Unani Medicine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Part 05.Indd
PART MISCELLANEOUS 5 TOPICS Awards and Honours Y NATIONAL AWARDS NATIONAL COMMUNAL Mohd. Hanif Khan Shastri and the HARMONY AWARDS 2009 Center for Human Rights and Social (announced in January 2010) Welfare, Rajasthan MOORTI DEVI AWARD Union law Minister Verrappa Moily KOYA NATIONAL JOURNALISM A G Noorani and NDTV Group AWARD 2009 Editor Barkha Dutt. LAL BAHADUR SHASTRI Sunil Mittal AWARD 2009 KALINGA PRIZE (UNESCO’S) Renowned scientist Yash Pal jointly with Prof Trinh Xuan Thuan of Vietnam RAJIV GANDHI NATIONAL GAIL (India) for the large scale QUALITY AWARD manufacturing industries category OLOF PLAME PRIZE 2009 Carsten Jensen NAYUDAMMA AWARD 2009 V. K. Saraswat MALCOLM ADISESHIAH Dr C.P. Chandrasekhar of Centre AWARD 2009 for Economic Studies and Planning, School of Social Sciences, Jawaharlal Nehru University, New Delhi. INDU SHARMA KATHA SAMMAN Mr Mohan Rana and Mr Bhagwan AWARD 2009 Dass Morwal PHALKE RATAN AWARD 2009 Actor Manoj Kumar SHANTI SWARUP BHATNAGAR Charusita Chakravarti – IIT Delhi, AWARDS 2008-2009 Santosh G. Honavar – L.V. Prasad Eye Institute; S.K. Satheesh –Indian Institute of Science; Amitabh Joshi and Bhaskar Shah – Biological Science; Giridhar Madras and Jayant Ramaswamy Harsita – Eengineering Science; R. Gopakumar and A. Dhar- Physical Science; Narayanswamy Jayraman – Chemical Science, and Verapally Suresh – Mathematical Science. NATIONAL MINORITY RIGHTS MM Tirmizi, advocate – Gujarat AWARD 2009 High Court 55th Filmfare Awards Best Actor (Male) Amitabh Bachchan–Paa; (Female) Vidya Balan–Paa Best Film 3 Idiots; Best Director Rajkumar Hirani–3 Idiots; Best Story Abhijat Joshi, Rajkumar Hirani–3 Idiots Best Actor in a Supporting Role (Male) Boman Irani–3 Idiots; (Female) Kalki Koechlin–Dev D Best Screenplay Rajkumar Hirani, Vidhu Vinod Chopra, Abhijat Joshi–3 Idiots; Best Choreography Bosco-Caesar–Chor Bazaari Love Aaj Kal Best Dialogue Rajkumar Hirani, Vidhu Vinod Chopra–3 idiots Best Cinematography Rajeev Rai–Dev D Life- time Achievement Award Shashi Kapoor–Khayyam R D Burman Music Award Amit Tivedi. -
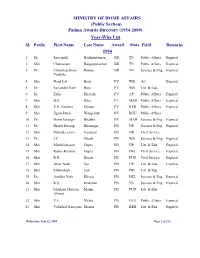
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

B4754.Pdf (2.181Mb)
Partners for Health in South-East Asia Conference Report New Delhi, India 16–18 March 2011 WHO Library Cataloguing-in-Publication data World Health Organization, Regional Offi ce for South-East Asia. Partners for health in South-East Asia: conference report. 1. Public-Private Sector Partnerships. 2. Health Services Administration. 3. Delivery of Health Care. 4. Health Status. 5. Maternal Mortality. 6. International Cooperation. 7. Health Planning. 8. Health priorities. 9. Communicable Diseases. ISBN 978-92-9022-405-1 (NLM classifi cation: WA 530) © World Health Organization 2011 All rights reserved. Requests for publications, or for permission to reproduce or translate WHO publications, whether for sale or for noncommercial distribution, can be obtained from Publishing and Sales, World Health Organization, Regional Offi ce for South-East Asia, Indraprastha Estate, Mahatma Gandhi Marg, New Delhi-110 002, India (fax: +91-11- 23370197; e-mail: publications@ searo.who.int). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specifi c companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Ref No: /SBICTCL/DT/2020-21 June 22, 2020 To, SBICAP Trustee
Ref No: /SBICTCL/DT/2020-21 June 22, 2020 To, SBICAP Trustee Company Limited Apeejay House, 6th Floor, West Wing, Dinshaw Wachha Road, Churchgate, Mumbai - 400 020 Dear Sir/Madam, Sub: Quarterly Compliance Report for the period January 1, 2020 to March 31, 2020. Re: Debenture Trust Deeds for various series of Bonds/Debentures issued – Statutory Compliance – Periodical Report for the quarter ending March 31, 2020 Pursuant to the Debenture Trust Deed dated May 26, 2017, January 18, 2018 May 10, 2019 and June 1, 2020 enclosed are the following:- 1. Names and address of Debenture holders with respective ISINs as on March 31, 2020 (enclosed as Annexure – 1); 2. We hereby confirm that: a. The Company has not defaulted in re-payment of principal and payment of interest to the debenture holders during the quarter ended March 31, 2020; b. That the assets of the Company on which the charge is created are adequate to discharge the obligations of the Company in respect of the Non- convertible Debentures (“NCDs”) issued by the Company - Asset Cover certificate will be provided once the annual audited financial results are approved in the Board Meeting. c. The properties charged for securing the issue of the NCDs are adequately insured and the policy is in the joint names of the Company and the Trustees; d. The Company has utilized the money raised by issue of Secured Redeemable NCDs for the purposes mentioned in the Disclosure Documents - Utilization certificate will be provided once the annual audited financial results are approved in the Board Meeting. e. -

Report 109 Commttee on Health and Family Welfare
REPORT NO. 109 PARLIAMENT OF INDIA RAJYA SABHA DEPARTMENT-RELATED PARLIAMENTARY STANDING COMMITTEE ON HEALTH AND FAMILY WELFARE ONE HUNDRED NINTH REPORT The National Medical Commission Bill, 2017 (Presented to the Rajya Sabha on20 th March, 2018 ) (Laid on the Table of Lok Sabha on20 th March, 2018 ) Rajya Sabha Secretariat, New Delhi March, 2018/Phalguna, 1939 (Saka) Website : http://rajyasabha.nic.in E-mail : [email protected] 117 Hindi version of this publication is also available PARLIAMENT OF INDIA RAJYA SABHA DEPARTMENT-RELATED PARLIAMENTARY STANDING COMMITTEE ON HEALTH AND FAMILY WELFARE ONE HUNDRED NINTH REPORT The National Medical Commission Bill, 2017 (Presented to the Rajya Sabha on 20th March, 2018) (Laid on the Table of Lok Sabha on 20th March, 2018) Rajya Sabha Secretariat, New Delhi March, 2018/ Phalguna, 1939 (Saka) CONTENTS PAGES 1. COMPOSITION OF THE COMMITTEE ....................................................................................... (i)-(ii) 2. INTRODUCTION ...................................................................................................................... (iii)-(iv) 3. ACRONYMS ............................................................................................................................ (v)-(vii) 4. REPORT ................................................................................................................................. 1-67 5. RECOMMENDATIONS/OBSERVATIONS — AT A GLANCE .......................................................... 68-82 6. MINUTES .............................................................................................................................. -

Report of the National Commission on Macroeconomics and Health Report of the National Commission on Macroeconomics and Health
Report of the National Commission on Macroeconomics and Health Report of the National Commission on Macroeconomics and Health Report of the National Commission on Macroeconomics and Health Report of the Report of the National Commission on Macroeconomics and Health Report of the National Commission on Macroeconomics and Health National Commission on Report of the Macroeconomics and Health National Commission on Macroeconomics and Health MINISTRY OF HEALTH AND FAMILY WELFARE GOVERNMENT OF INDIA, 2005 EQUITABLE DEVELOPMENT • HEALTHY FUTURE Report of the National Commission on Macroeconomics and Health National Commission on Macroeconomics and Health Ministry of Health & Family Welfare Government of India, New Delhi August 2005 © Ministry of Health & Family Welfare, Government of India September 2005 ISBN 81-7525-633-8 This Report does not address tertiary care and related areas such as super speciality hospital development in the public or private sector, telemedicine, medical tourism, environmental pollution or food safety etc. though they are all equally important. The Commission Report is based on background papers which can be accessed from the NCMH website www.mohfw.nic.in. They have also been published in two companion volumes. This report was written during the period April 1, 2004 - March 31, 2005. Printed at: Cirrus Graphics Private Limited B 261, Phase I, Naraina Industrial Area, New Delhi 110 028 Tel: + 91 11 51411507/1508 Fax: +91 11 51417575 email: [email protected] Editors: Pranay G. Lal and Byword Editorial Consultants Cover design: Quote Design Studio ii REPORT OF THE NATIONAL COMMISSION ON MACROECONOMICS AND HEALTH Members of the National Commission on Macroeconomics & Health Shri P. -

List of Fellows (Name-Wise) Upto 2016
LIST OF FELLOWS (NAME-WISE) UPTO 2016 0. Description Year 1. Abdul Kalam, A.P.J. Biomedical Engineering July 1995 DMIT. Former President, Republic of India. Res: 10 Rajaji Marg, New Delhi-110001. Permanent Address: No. 2, Mosque Street, Rameswaram, Ramanathapuram District, Tamil Nadu-623526. Tel: Off: (011) 3015321, 3014930, Res: (04567) 6493708, Fax: 2300756, E-mail: [email protected] (b 1931) (d.2015) Gen. Amir Chand Oration (NAMS, 1997-98) Padma Bhushan (1981); Padma Vibhushan (1990); Bharat Ratna (1997); D.Sc (h.c.) from several Universities; National Design Award; Dr. Biren Roy Space Award; Om Prakash Bhasin Award; National Nehru Award by Govt. of Madhya Pradesh; GM Modi Award for Science 1996; HK Firodia Award for Excellence in S&T 1996; Veer Savarkar Award 1998; Hon Fellow-Institution of Electronics and Telecommunication Engineers. 2. Abraham, Jacob Neurosurgery 1984 MS, MS (Neuro), FACS, FACA. Res: 10, 15th Avenue, Harrington Road, Chennai- 600031. Tel: Res: (044) 28363211, 42849258, Mobile: 09940118382, E-mail: [email protected] (b.1931). Basanti Devi Amir Chand Prize (ICMR, 1984); Sachs Memorial Lecturer, USA (1989). 3. Achari, Kamala Obstetrics and Gynecology 1982 MS, FRCOG, FICS, FACS. Emeritus Professor, Patna Medical College, Patna-800001 (Bihar). Res: 'Tirumalai', 21/D Road No.10, Rajendra Nagar, Patna- 800016. (b.1924) (d. 2014). 4. Adithan, C. Pharmacology July 2003 MD, PhD, FIMSA, FIPS. Former Professor & Head, Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education & Research, Pondicherry- 605006. Currently: Director-CIDRF and Professor of Pharmacology, Mahatma Gandhi Medical College and Research Institute, Pondicherry-607403. Res: Flat No. 1, Srinivas Towers, Vazhudavour Road, Kathirkamam, Pondicherry-605009. -

Indian Council of Medical Research New Delhi © 2005 Indian Council of Medical Research
Annual Report 2003-2004 Indian Council of Medical Research New Delhi © 2005 Indian Council of Medical Research Prof. N.K. Ganguly: Director-General Dr. K. Satyanarayana: Chief (Publication & Information) Complied and Edited by Dr. Sudha Chauhan, DDG (SG) Published by the Division of Publication, Information and Communication on behalf of the Director-General, Indian Council of Medical Research New Delhi 110029 Layout Design & Production Control by ICMR Press Unit Printed at: S. Narayan & Sons, B-88, Okhla Indl. Area, Phase-II, New Delhi Tel: 26385873 he Indian Council of Medical Research has made significant strides in its mandate of ‘Research for TBetter Health’. The Council has lived up to country’s expectations on all major fronts of its activity: research and development of vaccines and drugs for infectious diseases like tuberculosis, malaria, filariasis, HIV/AIDS, research in the areas of modern biology like molecular biology, genomics, bioinformatics, fertility regulation, cancer and other non-communicable diseases, consultancy and human resource development, national and international collaborations and biomedical informatics and communication. During the period under report, the Memorandum for the Expenditure Finance Committee (EFC) of ICMR has been approved by the Government of India and Rs.870 Crores have been allocated for the 10th Plan. The research activities will now be pursued with greater vigour as more funding would be available. To optimize resource utilization, the Council is using the Combined Approach Matrix being used by Global Health Forum for Health Research for setting research priorities in various disciplines. The Council continued to play an important role in detecting new and emerging infections in India like the Chandipura virus encephalitis in Andhra Pradesh and Gujarat and outbreak of mysterious fever in Siliguri. -

The India Cements Limited
THE INDIA CEMENTS LIMITED UNCLAIMED DIVIDEND FOR THE YEAR 2010-11 TO BE TRANSFERRED TO INVESTOR EDUCATION AND PROTECTION FUND AS REQUIRED UNDER SECTION 124 OF THE COMPANIES ACT 2013 READ WITH THE INVESTOR EDUCATION AND PROTECTION FUND AUTHORITY (ACCOUNTING, AUDIT, TRANSFER AND REFUND) RULES, 2016, AS AMENDED FOLIO / DPID_CLID NAME CITY PINCODE 1201040000010433 KUMAR KRISHNA LADE BHILAI 490006 1201060000057278 Bhausaheb Trimbak Pagar Nasik 422005 1201060000138625 Rakesh Dutt Panvel 410206 1201060000175241 JAI KISHAN MOHATA RAIPUR 492001 1201060000288167 G. VINOD KUMAR JAIN MANDYA 571401 1201060000297034 MALLAPPA LAGAMANNA METRI BELGAUM 591317 1201060000309971 VIDYACHAND RAMNARAYAN GILDA LATUR 413512 1201060000326174 LEELA K S UDUPI 576103 1201060000368107 RANA RIZVI MUZAFFARPUR 842001 1201060000372052 S KAILASH JAIN BELLARY 583102 1201060000405315 LAKHAN HIRALAL AGRAWAL JALNA 431203 1201060000437643 SHAKTI SHARAN SHUKLA BHADOHI 221401 1201060000449205 HARENDRASINH LALUBHA RANA LATHI 365430 1201060000460605 PARASHURAMAPPA A SHIMOGA 577201 1201060000466932 SHASHI KAPOOR BHAGALPUR 812001 1201060000472832 SHARAD GANESH KENI RATNAGIRI 415612 1201060000507881 NAYNA KESHAVLAL DAVE NALLASOPARA (E) 401209 1201060000549512 SANDEEP OMPRAKASH NEVATIA MAHAD 402301 1201060000555617 SYED QUAMBER HUSSAIN MUZAFFARPUR 842001 Page 1 of 301 FOLIO / DPID_CLID NAME CITY PINCODE 1201060000567221 PRASHANT RAMRAO KONDEBETTU BELGAUM 591201 1201060000599735 VANDANA MISHRA ALLAHABAD 211016 1201060000630654 SANGITA AGARWAL CUTTACK 753004 1201060000646546 SUBODH T -

Social Science TABLE of CONTENTS
2015 Social Science TABLE OF CONTENTS Academic Tools 79 Labour Economics 71 Agrarian Studies & Agriculture 60 Law & Justice 53 Communication & Media Studies 74-78 Literature 13-14 Counselling & Psychotherapy 84 7LHJL *VUÅPJ[:[\KPLZ 44-48 Criminology 49 Philosophy 24 Cultural Studies 9-13 Policy Studies 43 Dalit Sociology 8 Politics & International Relations 31-42 Development Communication 78 Psychology 80-84 Development Studies 69-70 Research Methods 94-95 Economic & Development Studies 61-69 SAGE Classics 22-23 Education 89-92 SAGE Impact 72-74 Environment Studies 58-59 SAGE Law 51-53 Family Studies 88 SAGE Studies in India’s North East 54-55 Film & Theatre Studies 15-18 Social Work 92-93 Gender Studies 19-21 Sociology & Social Theory 1-7 Governance 50 Special Education 88 Health & Nursing 85-87 Sport Studies 71 History 25-30 Urban Studies 56-57 Information Security Management 71 Water Management 59 Journalism 79 Index 96-100 SOCIOLOGY & SOCIAL THEORY HINDUISM IN INDIA A MOVING FAITH Modern and Contemporary Movements Mega Churches Go South Edited by Will Sweetman and Aditya Malik Edited by Jonathan D James Edith Cowan University, Perth Hinduism in India is a major contribution towards ongoing debates on the nature and history of the religion In A Moving Faith by Dr Jonathan James, we see for in India. Taking into account the global impact and the first time in a single coherent volume, not only that influence of Hindu movements, gathering momentum global Christianity in the mega church is on the rise, even outside of India, the emphasis is on Hinduism but in a concrete way, we are able to observe in detail as it arose and developed in sub-continent itself – an what this looks like across a wide variety of locations, approach which facilitates greater attention to detail cultures, and habitus. -

Annual Report 2012-13
From the Chairman's Pen 03 From the President's Pen 05 The Public Health Foundation of India 09 ?Who We Are 10 ?Our Collaborations 21 National Collaborations International Collaborations The PHFI logo is an engaging representation symbolic of good health, happiness, positive energy, renewal and collaborative change. The sunflower The Year Gone by…. 27 symbolises Public Health, and its petals represent flowering of its multiple disciplines and coming together of all stakeholders to fulfill the common goal Thematic Areas of Focus of working towards a healthier India. Just as the sunflower turns to the sun for ?Capacity Building 31 life-giving light, PHFI seeks to invigorate the Indian health system by ?Health Systems, Policy and Finance 53 knowledge generation, dissemination and its application for action to ?Non-Communicable Diseases 73 advance public health. The spiral in the logo depicts the bud of knowledge ?Infectious Diseases 91 Women and Child Health 97 blossoming into the flower of action. Vibrant orange is the colour of vitality, ? ?Public Health Nutrition 103 white symbolises truth and green symbolises the harmonious relation to the ?Affordable Health Technology 113 environment. ?Social Determinants of Health 117 Publications 127 Financials From the Chairman's Pen 03 From the President's Pen 05 The Public Health Foundation of India 09 ?Who We Are 10 ?Our Collaborations 21 National Collaborations International Collaborations The PHFI logo is an engaging representation symbolic of good health, happiness, positive energy, renewal and collaborative change. The sunflower The Year Gone by…. 27 symbolises Public Health, and its petals represent flowering of its multiple disciplines and coming together of all stakeholders to fulfill the common goal Thematic Areas of Focus of working towards a healthier India. -

National Academy of Medical Sciences (India)
NATIONAL ACADEMY OF MEDICAL SCIENCES (INDIA) ANNUAL REPORT 2015-16 NAMS House Ansari Nagar, Mahatma Gandhi Marg, New Delhi - 110029 Postal Address: NATIONAL ACADEMY OF MEDICAL SCIENCES (INDIA) NAMS House, Ansari Nagar, Mahatma Gandhi Marg, New Delhi – 110029 Telephone: 011-26588718 President : 011-26588792 Secretary : 011-26589289 Fax No.: 011-26588992 E-mail: [email protected] Website: http://nams-india.in CONTENTS Page No. The Council ─ 2015-16 1 Officers & Executive Staff 2 Editorial Board of Annals of NAMS 3 A. Organizational Activities 5 Organisational Activities & Annual Meeting 7 Award of Fellowships & Memberships at the Annual Meeting 8-17 Lifetime Achievement Award for the year 2014 18 Orations and Awards 19-24 NAMS 2007 Amritsar Award 25 Prof. J.S. Bajaj Award 25 Meetings of the Council 26 Election of Fellows – 2015 26-30 Election of Members – 2015 31-40 Candidates proposed for Membership (MNAMS) on passing DNB Examination 41-48 Fellows/Members on rolls of the Academy 49 Nominations of Medical Scientists for Orations and Awards – 2015-16 50-54 Golden Jubilee Commemoration Award Lecture 55 Lifetime Achievement Award for the year 2015 55 Maintenance of Building 55-56 Publication of Annals 57-59 Obituary 60 Text of the address by the President Dr. Mukund Sadashiv Joshi, delivered at the Annual Convocation on 17th October, 2015 at Patna, Bihar (Annexure I) 61 Text of the address by the Chief Guest Dr. Ram Nath Kovind, Governor of Bihar, GoI, at the Annual Convocation on 17th October 2015, at Patna (Annexure II) 62-63 B. Academic Report 65 Continuing Medical Education Programme 67 Report of activities April 1, 2015 - March 31, 2016 67-71 State-wise distribution of Extramural CME Programmes 72 Extramural CME Programmes of NAMS Chapters (Annexure III) 73-74 Report on Extramural CME Programmes (Annexure IV) 75-87 The Medical Scientists’ Exchange Programmes (Health Manpower Development) 88 Report on Intramural CME Programmes (Annexure V) 89-96 NAMS Chapters (Annexure VI) 97-100 Emeritus Professors of NAMS 101-103 NAMS Website 104 C.