Parapharyngeal Space Tumors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
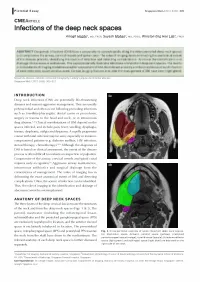
Infections of the Deep Neck Spaces
!Pictorial Essay Singapore Med J 2012; 53(5) 305 CM EARTICLE Infections of the deep neck spaces Amogh Hegdel, MD, FRCR, Suyash Mohan2, MD, PDCC, Winston Eng Hoe Lima, FRCR ABSTRACT Deep neck infections (DNI) have a propensity to spread rapidly along the interconnected deep neck spaces and compromise the airway, cervical vessels and spinal canal. The value of imaging lies in delineating the anatomical extent of the disease process, identifying the source of infection and detecting complications. Its role in the identification and drainage of abscesses is well known. This paper pictorially illustrates infections of important deep neck spaces. The merits and drawbacks of imaging modalities used for assessment of DNI, the relevant anatomy and the possible sources of infection of each deep neck space are discussed. Certain imaging features that alter the management of DNI have been highlighted. Keywords: abscess, cellulitis, computed tomography, Ludwig's angina, peritonsillar abscess Singapore Med J2012; 53(5): 305-312 INTRODUCTION la Deep neck infections (DNI) are potentially life -threatening diseases and warrant aggressive management. They are usually polymicrobial and often occur following preceding infections such as tonsillitis/pharyngitis, dental caries or procedures, surgery or trauma to the head and neck, or in intravenous drug abusers.(1,2) Clinical manifestations of DNI depend on the EJ spaces infected, and include pain, fever, swelling, dysphagia, trismus, dysphonia, otalgia and dyspnoea. A rapidly progressive course with fatal outcome may be seen, especially in immuno- compromised patients (e.g. diabetes mellitus, HIV infection, steroid therapy, chemotherapy).(' -3) Although the diagnosis of DNI is based on clinical assessment, the extent of the disease process is often difficult to evaluate on inspection or palpation. -

Transoral Robotic Assisted Resection of the Parapharyngeal Space
UCLA UCLA Previously Published Works Title Transoral robotic assisted resection of the parapharyngeal space. Permalink https://escholarship.org/uc/item/0wh104s6 Journal Head & neck, 37(2) ISSN 1043-3074 Author Mendelsohn, Abie H Publication Date 2015-02-01 DOI 10.1002/hed.23724 Peer reviewed eScholarship.org Powered by the California Digital Library University of California OPERATIVE TECHNIQUES–PICTORIAL ESSAY Transoral robotic assisted resection of the parapharyngeal space Abie H. Mendelsohn, MD* Director - Robotic Head & Neck Surgery Program, Department of Head and Neck Surgery, David Geffen School of Medicine at University of California – Los Angeles, Los Angeles, California and Jonsson Comprehensive Cancer Center, David Geffen School of Medicine at University of California – Los Angeles, Los Angeles, California. Accepted 28 April 2014 Published online 15 November 2014 in Wiley Online Library (wileyonlinelibrary.com). DOI 10.1002/hed.23724 ABSTRACT: Background. Preliminary case series have reported clinical Results. Transoral robotic assisted resection of a 54- 3 46-mm paraphar- feasibility and safety of a transoral minimally invasive technique to yngeal space mass was performed, utilizing 97 minutes of robotic surgical approach parapharyngeal space masses. With the assistance of the sur- time. Pictorial demonstration of the robotic resection is provided. gical robotic system, tumors within the parapharyngeal space can now Conclusion. Parapharyngeal space tumors have traditionally been be excised safely without neck incisions. A detailed technical description approached via transcervical skin incisions, typically including blunt dissection is included. from tactile feedback. The transoral robotic approach offers magnified 3D Methods. After developing compressive symptoms from a parapharyng- visualization of the parapharyngeal space that allows for complete and safe eal space lipomatous tumor, the patient was referred by his primary oto- resection. -

Head & Neck Surgery Course
Head & Neck Surgery Course Parapharyngeal space: surgical anatomy Dr Pierfrancesco PELLICCIA Pr Benjamin LALLEMANT Service ORL et CMF CHU de Nîmes CH de Arles Introduction • Potential deep neck space • Shaped as an inverted pyramid • Base of the pyramid: skull base • Apex of the pyramid: greater cornu of the hyoid bone Introduction • 2 compartments – Prestyloid – Poststyloid Anatomy: boundaries • Superior: small portion of temporal bone • Inferior: junction of the posterior belly of the digastric and the hyoid bone Anatomy: boundaries Anatomy: boundaries • Posterior: deep fascia and paravertebral muscle • Anterior: pterygomandibular raphe and medial pterygoid muscle fascia Anatomy: boundaries • Medial: pharynx (pharyngobasilar fascia, pharyngeal wall, buccopharyngeal fascia) • Lateral: superficial layer of deep fascia • Medial pterygoid muscle fascia • Mandibular ramus • Retromandibular portion of the deep lobe of the parotid gland • Posterior belly of digastric muscle • 2 ligaments – Sphenomandibular ligament – Stylomandibular ligament Aponeurosis and ligaments Aponeurosis and ligaments • Stylopharyngeal aponeurosis: separates parapharyngeal spaces to two compartments: – Prestyloid – Poststyloid • Cloison sagittale: separates parapharyngeal and retropharyngeal space Aponeurosis and ligaments Stylopharyngeal aponeurosis Muscles stylohyoidien Stylopharyngeal , And styloglossus muscles Prestyloid compartment Contents: – Retromandibular portion of the deep lobe of the parotid gland – Minor or ectopic salivary gland – CN V branch to tensor -

Deep Neck Infections 55
Deep Neck Infections 55 Behrad B. Aynehchi Gady Har-El Deep neck space infections (DNSIs) are a relatively penetrating trauma, surgical instrument trauma, spread infrequent entity in the postpenicillin era. Their occur- from superfi cial infections, necrotic malignant nodes, rence, however, poses considerable challenges in diagnosis mastoiditis with resultant Bezold abscess, and unknown and treatment and they may result in potentially serious causes (3–5). In inner cities, where intravenous drug or even fatal complications in the absence of timely rec- abuse (IVDA) is more common, there is a higher preva- ognition. The advent of antibiotics has led to a continu- lence of infections of the jugular vein and carotid sheath ing evolution in etiology, presentation, clinical course, and from contaminated needles (6–8). The emerging practice antimicrobial resistance patterns. These trends combined of “shotgunning” crack cocaine has been associated with with the complex anatomy of the head and neck under- retropharyngeal abscesses as well (9). These purulent col- score the importance of clinical suspicion and thorough lections from direct inoculation, however, seem to have a diagnostic evaluation. Proper management of a recog- more benign clinical course compared to those spreading nized DNSI begins with securing the airway. Despite recent from infl amed tissue (10). Congenital anomalies includ- advances in imaging and conservative medical manage- ing thyroglossal duct cysts and branchial cleft anomalies ment, surgical drainage remains a mainstay in the treat- must also be considered, particularly in cases where no ment in many cases. apparent source can be readily identifi ed. Regardless of the etiology, infection and infl ammation can spread through- Q1 ETIOLOGY out the various regions via arteries, veins, lymphatics, or direct extension along fascial planes. -

ODONTOGENTIC INFECTIONS Infection Spread Determinants
ODONTOGENTIC INFECTIONS The Host The Organism The Environment In a state of homeostasis, there is Peter A. Vellis, D.D.S. a balance between the three. PROGRESSION OF ODONTOGENIC Infection Spread Determinants INFECTIONS • Location, location , location 1. Source 2. Bone density 3. Muscle attachment 4. Fascial planes “The Path of Least Resistance” Odontogentic Infections Progression of Odontogenic Infections • Common occurrences • Periapical due primarily to caries • Periodontal and periodontal • Soft tissue involvement disease. – Determined by perforation of the cortical bone in relation to the muscle attachments • Odontogentic infections • Cellulitis‐ acute, painful, diffuse borders can extend to potential • fascial spaces. Abscess‐ chronic, localized pain, fluctuant, well circumscribed. INFECTIONS Severity of the Infection Classic signs and symptoms: • Dolor- Pain Complete Tumor- Swelling History Calor- Warmth – Chief Complaint Rubor- Redness – Onset Loss of function – Duration Trismus – Symptoms Difficulty in breathing, swallowing, chewing Severity of the Infection Physical Examination • Vital Signs • How the patient – Temperature‐ feels‐ Malaise systemic involvement >101 F • Previous treatment – Blood Pressure‐ mild • Self treatment elevation • Past Medical – Pulse‐ >100 History – Increased Respiratory • Review of Systems Rate‐ normal 14‐16 – Lymphadenopathy Fascial Planes/Spaces Fascial Planes/Spaces • Potential spaces for • Primary spaces infectious spread – Canine between loose – Buccal connective tissue – Submandibular – Submental -

Endocrine Tumors Associated with the Vagus Nerve
239 A Varoquaux et al. Vagal endocrine tumors 23:9 R371–R379 Review Endocrine tumors associated with the vagus nerve Arthur Varoquaux1, Electron Kebebew2, Fréderic Sebag3, Katherine Wolf4, Jean-François Henry3, Karel Pacak4 and David Taïeb5 1Department of Radiology, Conception Hospital, Aix-Marseille University, Marseille, France 2Endocrine Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA 3Department of Endocrine Surgery, Conception Hospital, Aix-Marseille University, Marseille, France Correspondence 4Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human should be addressed Development (NICHD), National Institutes of Health, Bethesda, Maryland, USA to D Taïeb 5Department of Nuclear Medicine, La Timone University Hospital, CERIMED, Aix-Marseille University, Marseille, France Email [email protected] Abstract The vagus nerve (cranial nerve X) is the main nerve of the parasympathetic division of Key Words the autonomic nervous system. Vagal paragangliomas (VPGLs) are a prime example f vagus nerve of an endocrine tumor associated with the vagus nerve. This rare, neural crest tumor f paragangliomas constitutes the second most common site of hereditary head and neck paragangliomas f hyperparathyroidism (HNPGLs), most often in relation to mutations in the succinate dehydrogenase complex f diagnostic imaging subunit D (SDHD) gene. The treatment paradigm for VPGL has progressively shifted from surgery to abstention or therapeutic radiation with curative-like outcomes. Parathyroid tissue and parathyroid adenoma can also be found in close association with the vagus Endocrine-Related Cancer Endocrine-Related nerve in intra or paravagal situations. Vagal parathyroid adenoma can be identified with preoperative imaging or suspected intraoperatively by experienced surgeons. -

Perinatal (Fetal and Neonatal) Astrocytoma: a Review
Childs Nerv Syst DOI 10.1007/s00381-016-3215-y REVIEW PAPER Perinatal (fetal and neonatal) astrocytoma: a review Hart Isaacs Jr.1,2 Received: 16 July 2016 /Accepted: 3 August 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Keywords Fetal astrocytoma . Neonatal astrocytoma . Introduction The purpose of this review is to document the Perinatal astrocytoma . Intracranial hemorrhage . Congenital various types of astrocytoma that occur in the fetus and neo- brain tumor nate, their locations, initial findings, pathology, and outcome. Data are presented that show which patients are likely to sur- vive or benefit from treatment compared with those who are Introduction unlikely to respond. Materials and methods One hundred one fetal and neonatal Glial cells are the supportive elements of the central nervous tumors were collected from the literature for study. system (CNS) [22]. They include astrocytes, oligodendro- Results Macrocephaly and an intracranial mass were the most cytes, and ependymal cells, and the corresponding tumors common initial findings. Overall, hydrocephalus and intracra- originating from these cells astrocytoma, oligodendroglioma, nial hemorrhage were next. Glioblastoma (GBM) was the and ependymoma all of which are loosely called Bglioma^ most common neoplasm followed in order by subependymal [16, 22]. The term Bglioma^ is used interchangeably with giant cell astrocytoma (SEGA), low-grade astrocytoma, ana- astrocytoma to describe the more common subgroup of tu- plastic astrocytoma, and desmoplastic infantile astrocytoma mors [22]. (DIA). Tumors were detected most often toward the end of Glioma (astrocytoma) is the leading CNS tumor in chil- the third trimester of pregnancy. -

Surgical Treatment of Benign Parapharyngeal Space Tumours
Med Oral Patol Oral Cir Bucal. 2008 Jan1;13(1):E61-4. Treatment of benign parapharyngeal space tumours Med Oral Patol Oral Cir Bucal. 2008 Jan1;13(1):E61-4. Treatment of benign parapharyngeal space tumours Surgical treatment of benign parapharyngeal space tumours. Presentation of two clinical cases and revision of the literature Martin Fernández Ferro1, Jacinto Fernández Sanromán 2, Alberto Costas López 1, Jesús Sandoval Gutierrez 1, Annahys López de Sanchez 1 (1) Medico Adjunto (2) Jefe de Servicio. Servicio de Cirugía Oral y Maxilofacial, Hospital Povisa. Vigo Correspondence: Dr. Martín Fernández Ferro Servicio de Cirugía Oral y Maxilofacial Hospital Povisa. C/ Salamanca 5, 36211. Vigo. Spain E-mail: [email protected] Fernández-Ferro M, Fernández-Sanromán J, Costas-López A, Sandoval- Gutierrez J, López-de Sanchez A. Surgical treatment of benign parapha- ryngeal space tumours. Presentation of two clinical cases and revision of Received: 5-05-2007 Accepted: 17-06-2007 the literature. Med Oral Patol Oral Cir Bucal. 2008 Jan1;13(1):E61-4. © Medicina Oral S. L. C.I.F. B 96689336 - ISSN 1698-6946 http://www.medicinaoral.com/medoralfree01/v13i1/medoralv13i1p61.pdf Indexed in: -Index Medicus / MEDLINE / PubMed -EMBASE, Excerpta Medica -SCOPUS -Indice Médico Español -IBECS Abstract Parapharyngeal space (PPS) tumours, most of them benign, account for some 0.5% of tumours of the head and neck. The importance of these tumours lies mainly in two aspects: on the one hand, the difficulty of early diagnosis, due to the lack of symptoms in the initial stages and, on the other, the extreme complications of performing surgery in the parapharyngeal region. -

Target Volume Definition Guidelines
Target Volume Definition Guidelines 1 INTRODUCTION Accurate target volume definition is an absolute requirement of radiotherapy planning. 3-Dimensional Conformal Radiotherapy (3D-CRT) and, in particular, Intensity Modulated Radiotherapy (IMRT), require detailed knowledge of CT- based anatomy. IMRT allows the delivery of very precise dose distributions, so that areas not specifically included in the target volume will not be treated to a therapeutic dose. Therefore, great care must be taken to ensure all the involved areas and those at risk are included. The application of 3D-CRT and IMRT to head and neck cancers has resulted in standardised guidelines for the CT delineation of clinical target volumes (CTV) in the node negative neck, node positive neck and postoperative neck [1] . Clinical target volume definition of tumours of hypopharynx and oropharynx have also been proposed in the guidelines for the PARSPORT Trial[2]. This document describes target volume definition of the parotid bed and indications for ipsilateral neck irradiation for patients entering the COSTAR trial. 2 ANATOMY OF THE PAROTID GLAND The parotid gland is the largest salivary gland. It measures approximately 6cm x 3.5cm. It is an irregular wedge shaped unilobular organ with autonomic innervation and encapsulating the facial nerve. Figure 1: Major salivary glands Anatomical borders • tragus • midpoint masseter • angle of mandible • mastoid process It has five compartments: 3 superficial and 2 deep, all within a capsule in continuum with the deep cervical fascia. Stensen’s duct is the parotid duct which lies anterior and exits the buccal mucosa at the level of the second upper molar. 3 Parotid Figure 2: MRI axial image of right parotid tumour The parotid gland lies in the parotid compartment which is contained within the following anatomical landmarks: • Superior- zygomatic arch • Inferior- styloid process • Anterior- anterior border of masseter • Posterior- mastoid process Pathology Malignant parotid tumours are commonly classified as low grade and high grade. -

Surgical Strategy in the Transcervical Approach of the Parapharyngeal Tumors in Aeronautical Military Personnel
Review of the Air Force Academy No 1 (28) 2015 SURGICAL STRATEGY IN THE TRANSCERVICAL APPROACH OF THE PARAPHARYNGEAL TUMORS IN AERONAUTICAL MILITARY PERSONNEL Cristian Dragos STEFANESCU*, Viorel ZAINEA**, Mura HAINAROSIE**, Razvan HAINAROSIE** *“Gen. Dr. Aviator Victor Anastasiu” National Institute of Aeronautical and Space Medicine, **„Carol Davila” University of Medicine and Pharmacy, Bucharest Abstract: The parapharyngeal space is an anatomic region that may present various forms of tumoral masses, different by histology, extension and location. For ENT aeromedical examinator, deciding the most proper surgical approach in this treatment may be difficult. The authors analyze diagnostic and treatment criteria used for parapharyngeal space tumors and review the surgical techniques used. Transcervical approach is evaluated. Keywords: trancervical, mandibulotomy ,parapharyngealspace, surgical approach. INTRODUCTION Usualy, considering the anatomic component of the two regions of the parapharyngeal The anatomy of the parapharyngeal space space, tumoral differential diagnostic may be is well known. It has the shape of a pyramid, suggested my tumor location. based upward to the base of the skull, with the The most usual tumors found in the prestylod top oriented to the hyoid bone’s great horn. region are pleomorphic adenoma of the parotid, The pyramid is divided into two separate imflamatory lymphatic nodes, methastatic regions. Anteriorly, the presyloid region lymph nodes, lymphoma, ectopic thyroid. In consists of the deeplobeof theparotid gland, the retrostyloid region we usualy expect to find internalmaxillaryartery, inferioralveolarnerve, paragangliomas, schwannomas, solitary fibrous lingual,auriculo-temporal and fat. Posteriorly, tumors. the retrostyloid region includes the neuro- vascular package of the neck with the internal MATERIAL AND METHODS carotid artery, internal jugular vein, cranial nervesglossopharyngeal, vagus, accessory, The authors review the most usual hypoglossalandcervicalsympatheticchain. -

Cytogenetics and Molecular Genetics of Childhood Brain Tumors 1
Neuro-Oncology Cytogenetics and molecular genetics of childhood brain tumors 1 Jaclyn A. Biegel 2 Division of Human Genetics and Molecular Biology, The Children’s Hospital of Philadelphia and the Department of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA 19104 Considerable progress has been made toward improving Introduction survival for children with brain tumors, and yet there is still relatively little known regarding the molecular genetic Combined cytogenetic and molecular genetic approaches, events that contribute to tumor initiation or progression. including preparation of karyotypes, FISH, 3 CGH, and Nonrandom patterns of chromosomal deletions in several loss of heterozygosity studies have led to the identica- types of childhood brain tumors suggest that the loss or tion of regions of the genome that contain a variety of inactivation of tumor suppressor genes are critical events novel tumor suppressor genes and oncogenes. Linkage in tumorigenesis. Deletions of chromosomal regions 10q, analysis in families, which segregate the disease pheno- 11 and 17p, for example, are frequent events in medul- type, and studies of patients with constitutional chromo- loblastoma, whereas loss of a region within 22q11.2, somal abnormalities have resulted in the identication of which contains the INI1 gene, is involved in the develop- many of the disease genes for which affected individuals ment of atypical teratoid and rhabdoid tumors. A review have an inherited predisposition to brain tumors (Table of the cytogenetic and molecular genetic changes identi- 1). The frequency of mutations of these genes in sporadic ed to date in childhood brain tumors will be presented. tumors, however, is still relatively low. -

Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors
The American Journal of Pathology, Vol. 188, No. 6, June 2018 ajp.amjpathol.org ANIMAL MODELS Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors Morgan L. Shannon,* Ryann M. Fame,* Kevin F. Chau,*z Neil Dani,* Monica L. Calicchio,* Gwenaelle S. Géléoc,x{ Hart G.W. Lidov,* Sanda Alexandrescu,* and Maria K. Lehtinen*z From the Departments of Pathology* and Otolaryngologyx and the F.M. Kirby Center for Neurobiology,{ Boston Children’s Hospital, Boston; and the Program in Biological and Biomedical Sciences,z Harvard Medical School, Boston, Massachusetts Accepted for publication February 20, 2018. Choroid plexus tumors and ciliary body medulloepithelioma are predominantly pediatric neoplasms. Progress in understanding the pathogenesis of these tumors has been hindered by their rarity and lack Address correspondence to fi Maria K. Lehtinen, Ph.D., of models that faithfully recapitulate the disease. Here, we nd that endogenous Myc proto-oncogene Department of Pathology, Boston protein is down-regulated in the forebrain neuroepithelium, whose neural plate border domains give Children’s Hospital, 300 Long- rise to the anterior choroid plexus and ciliary body. To uncover the consequences of persistent Myc wood Ave., Boston, MA 02115. expression, MYC expression was forced in multipotent neural precursors (nestin-Cre:Myc), which E-mail: maria.lehtinen@ produced fully penetrant models of choroid plexus carcinoma and ciliary body medulloepithelioma. childrens.harvard.edu. Nestin-mediated MYC expression in the epithelial cells of choroid plexus leads to the regionalized formation of choroid plexus carcinoma in the posterior domain of the lateral ventricle choroid plexus and the fourth ventricle choroid plexus that is accompanied by loss of multiple cilia, up-regulation of protein biosynthetic machinery, and hydrocephalus.