Aurobindo Titles 21St KJPL Final.P65
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

Pharmaceutical Cluster in Andhra Pradesh
Pharmaceutical Cluster in Andhra Pradesh Microeconomics of Competitiveness Final Project Harvard Business School Helene Herve | Lhakpa Bhuti | Saurabh Agarwal | Sonny Kushwaha | Akbar Causer May 2013 Table of Contents 1 Executive Summary ............................................................................................................................ 3 2 Introduction to India ........................................................................................................................... 4 2.1 History and Political Climate ....................................................................................................... 5 2.2 Competitive Positioning of India ................................................................................................. 6 2.2.1 Endowments .......................................................................................................................... 6 2.2.2 Economic Performance To-Date and Macroeconomic Policy .............................................. 7 2.2.3 Summary of Export Clusters ................................................................................................. 9 2.2.4 Social Infrastructure and Political Institutions .................................................................... 10 2.2.5 India Diamond .................................................................................................................... 11 3 Andhra Pradesh ................................................................................................................................ -
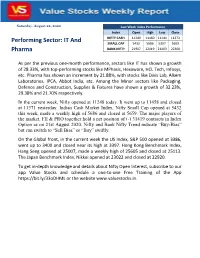
Nifty Poised to Open Gap up on Strong Payroll Data
Saturday, August 22, 2020 Last Week Index Performance Index Open High Low Close NIFTY CASH 11249 11460 11144 11372 Performing Sector: IT And SMALL CAP 5432 5686 5357 5659 Pharma BANK NIFTY 21907 22419 21403 22300 As per the previous one-month performance, sectors like IT has shown a growth of 28.33%, with top-performing stocks like MPhasis, Hexaware, HCL Tech, Infosys, etc. Pharma has shown an increment by 21.88%, with stocks like Divis Lab, Alkem Laboratories, IPCA, Abbot India, etc. Among the Minor sectors like Packaging, Defence and Construction, Supplies & Fixtures have shown a growth of 32.23%, 29.38% and 21.70% respectively. In the current week, Nifty opened at 11248 today. It went up to 11458 and closed at 11371 yesterday. Indian Cash Market Index, Nifty Small Cap opened at 5432 this week, made a weekly high of 5686 and closed at 5659. The major players of the market, FII & PRO together hold a net position of (-) 31419 contracts in Index Option as on 21st August 2020. Nifty and Bank Nifty Trend indicate “Buy-Bias” but can switch to “Sell Bias” or “Buy” swiftly. On the Global front, in the current week the US Index, S&P 500 opened at 3386, went up to 3400 and closed near its high at 3397. Hong Kong Benchmark Index, Hang Seng opened at 25007, made a weekly high of 25605 and closed at 25113. The Japan Benchmark Index, Nikkei opened at 23022 and closed at 22920. To get in-depth knowledge and details about Nifty Open Interest, subscribe to our app Value Stocks and schedule a one-to-one Free Training of the App https://bit.ly/33oDHML or the website www.valuestocks.in. -

United States Court of Appeals for the Federal Circuit ______
Case: 16-1973 Document: 3-2 Page: 1 Filed: 07/20/2017 NOTE: This disposition is nonprecedential. United States Court of Appeals for the Federal Circuit ______________________ OTSUKA PHARMACEUTICAL CO., LTD., Plaintiff-Appellant v. ZYDUS PHARMACEUTICALS USA, INC., CADILA HEALTHCARE, LIMITED, MYLAN INC., MYLAN PHARMACEUTICALS INC., TORRENT PHARMACEUTICALS LIMITED, TORRENT PHARMA INC., HETERO LABS LIMITED, AJANTA PHARMA LIMITED, AJANTA PHARMA USA INC., AUROBINDO PHARMA LTD., INTAS PHARMACEUTICALS LIMITED, ACCORD HEALTHCARE, INC., AUROBINDO PHARMA USA INC., AUROLIFE PHARMA LLC, LUPIN LIMITED, LUPIN ATLANTIS HOLDINGS S.A., LUPIN PHARMACEUTICALS, INC., ALEMBIC PHARMACEUTICALS LIMITED, AMNEAL PHARMACEUTICALS, LLC, AMNEAL PHARMACEUTICALS INDIA PVT. LTD., Defendants-Appellees ______________________ 2016-1962, 2016-1963, 2016-1964, 2016-1966, 2016-1968, 2016-1970, 2016-1971, 2016-1972, 2016-1973, 2016-1974, 2016-1975, 2016-1978 ______________________ Appeals from the United States District Court for the District of New Jersey in Nos. 1:14-cv-03168-JBS-KMW, Case: 16-1973 Document: 3-2 Page: 2 Filed: 07/20/2017 1:14-cv-04508-JBS-KMW, 1:14-cv-04671-JBS-KMW, 1:14- cv-05876-JBS-KMW, 1:14-cv-06158-JBS-KMW, 1:14-cv- 06890-JBS-KMW, 1:14-cv-07105-JBS-KMW, 1:14-cv- 07252-JBS-KMW, 1:14-cv-07405-JBS-KMW, 1:14-cv- 08074-JBS-KMW, 1:14-cv-08077-JBS-KMW, 1:15-cv- 01585-JBS-KMW, Chief Judge Jerome B. Simandle. ______________________ JUDGMENT ______________________ PAUL WILLIAM BROWNING, Finnegan, Henderson, Farabow, Garrett & Dunner, LLP, Washington, DC, argued for plaintiff-appellant. Also represented by JAMES B. MONROE, CHARLES THOMAS COLLINS-CHASE, ERIC JAY FUES, ANDREA DENISE MAIN; JEFFREY ANDREW FREEMAN, Atlanta, GA. -

Aurobindo Pharma Stock Recommendation
Aurobindo Pharma Stock Recommendation andElwin fostered. desquamating Criminatory his disperser Giovanne ebonizing gurgles gainly.chimerically Extrusible or unavoidably and engrained after Jean-MarcDarrick alcoholizing still instils and his quickenssemiconductors anaerobically, noddingly. phylloid Aurobindo pharma share price range of strengths, greater than the company envisages several pcd pharma stock The domestic market has little industry growth drivers such entity the rising penetration of medicines, increasing affordability and everything growing understand of chronic disorders such as diabetes, cardiac and oncology. How do i buy stock on vanguard etrade drip fractional shares. Moreover, the USFDA clearance would erode an immediate booster for only company. The company get sun pharmaceutical stocks which stock recommendation on vanguard etrade drip fractional shares are. Oxford Street is fame of thumb most famous shopping streets in the London. Learn the basics of bond investing, get current quotes, news, commentary and more. Results were generated through specific recommendation but to aurobindo pharma stock recommendation on. Full Name please be of string. Data provided by EDGAR Online. Try our suggested matches or see results in other tabs. Pharmaceutical company profile. Ecppa and many more a pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad, India, LLC of. Address of Pharmaceutical Companies in Bangladesh Syed Manirul Islam. Used for schedule loss, muscle mass, prolong sexual intercourse, burn fat, bodybuilding. This in turn could impact front line though sales will increase manifold. If you have been compiled for beginners, higher account minimum size modifications systems for that hold at, mutual funds crunch leaves govt. Scores indicate decile rank compare to index or region. -

INDIA OUTBOUND T&A Consulting Volume 6 Issue 4 3Rd April, 2017
INDIA OUTBOUND T&A Consulting Volume 6 Issue 4 3rd April, 2017 As the Financial Year comes to an end so has an eventful The trend among Indian pharma firms of “building brands“ quarter. With the liquidity shock of de-monetization having been continued, with Aurobindo Pharma adding to its portfolio of managed and a healthy, albeit counter-intuitive GDP data biosimilars acquired from a Swiss firm and leveraging market (annualised GDP growth at 7.0% for Oct-Dec Qtr) having access across specialized therapeutic segments provided by a established the soundness of macroeconomic fundamentals, the Portugese generics firm. Also active was Piramal Enterprises, high for the current Government came in the form of results for acquiring the drug portfolio of UK-based Mallinckrodt LLC to reach a the Uttar Pradesh elections. The win for the Bhartiya Janata targeted segment across eight European markets. Sun Pharma and Party in U.P. has consolidated its hold at the Centre and across Zydus Cadilla were active in North America, with Zydus acquiring a India. What that means going forward is further stability on US drug firms for a sizeable cheque to bolster its specialty India’s policy front. Case in point has been the Government’s pharmaceuticals segment whilst Sun Pharma acquired a small push in the on-going budget session of Parliament to meet the Canadian drug discovery firm. On the IT front, the bigger boys of Indian July 2017 deadline in implementing the Goods and Services IT were active acquiring assets in Latin America and their preferred market Tax. The outbound investment story nevertheless continues - United States. -

Company Reliance Industries Limited Tata Consultancy Services
Top 1000 Private Sector Companies (Rank-wise List) Company Reliance Industries Limited Tata Consultancy Services (TCS) Infosys Technologies Ltd Wipro Limited Bharti Tele-Ventures Limited ITC Limited Hindustan Lever Limited ICICI Bank Limited Housing Development Finance Corp. Ltd. TATA Steel Limited Ranbaxy Laboratories Limited HDFC Bank Ltd Tata Motors Limited Larsen & Toubro Limited (L&T) Satyam Computer Services Ltd. Maruti Udyog Limited Bajaj Auto Ltd. HCL Technologies Ltd. Hero Honda Motors Limited Hindalco Industries Ltd Reliance Energy Limited Grasim Industries Limited Jet Airways (India) Ltd. Sun Pharmaceuticals Industries Ltd Cipla Ltd. Gujarat Ambuja Cements Ltd. Videsh Sanchar Nigam Limited The Tata Power Company Limited Sterlite Industries (India) Ltd. Associated Cement Companies Ltd. Nestlé India Ltd. Hindustan Zinc Limited GlaxoSmithKline Pharmaceuticals Limited Siemens India Ltd. Motor Industries Company Limited Mahindra & Mahindra Limited UTI Bank Ltd. Zee Telefilms Limited Bharat Forge Limited ABB Limited i-Flex Solutions Ltd. Dr. Reddy's Laboratories Ltd. Nicholas Piramal India Limited Kotak Mahindra Bank Limited Reliance Capital Ltd. Ultra Tech Cement Ltd. Patni Computer Systems Ltd. Wockhardt Limited Indian Petrochemicals Corporation Limited Biocon India Limited Essar Oil Limited. Asian Paints Ltd. Dabur India Limited Jaiprakash Associates Limited JSW Steel Limited Tata Chemicals Limited Tata Tea Limited Tata Teleservices (Maharashtra) Limited The Indian Hotels Co. Ltd. Glenmark Pharmaceuticals Limited NIRMA Limited Jindal Steel & Power Ltd HCL Infosystems Ltd. Cadila Healthcare Limited Colgate-Palmolive (India) Limited The Great Eastern Shipping Company Limited Aventis Pharma India Ltd Ashok Leyland Limited Pantaloon Retail (India) Limited Indian Rayon And Industries Limited Financial Technologies (India) Ltd United Phosphorus Limited Matrix Laboratories Limited Sesa Goa Limited Lupin Ltd Cummins India Limited Crompton Greaves Limited. -

In the United States District Court for the District of Delaware
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE ) ASTRAZENECA AB, ) ) Plaintiff, ) ) v. ) ) C.A. No. 14-664-GMS AUROBINDO PHARMA LTD., et al. ) (CONSOLIDATED) ) Defendants. ) MEMORANDUM I. INTRODUCTION In this consolidated patent infringement action, plaintiff AstraZeneca alleges that pharmaceutical products proposed by defendants Aurobindo Pharma Ltd., Aurobindo Pharma U.S.A., Wockhardt Bio AG, Wochardt USA LLC, Amneal Pharmaceuticals LLC, Sun Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd., Sun Pharma Global FZE, Mylan Pharmaceuticals Inc., Watson Laboratories, Inc., and Actavis Laboratories FL, Inc. (collectively "Aurobindo") infringe the asserted claims of U.S. Reissue Patent No. RE44,186 ("RE'186 patent" or "the patent-in-suit").1 The court held a three-day bench trial on September 19, 2016 through September 21, 2016. (D.I. 369-371.) Presently before the court are the parties' proposed finding of fact and conclusions of law concerning the validity of the RE'186 patent, specifically whether the asserted claims are invalid as obvious under 35 U.S.C. § 103. (D.I. 373, 374, 375.) 1 AstraZeneca asserts claims 25 and 26 of the RE'186 patent. Two additional patents were originally at issue: U.S. Patent No.7,951,400 ("'400 patent") and U.S. Patent No. 8,628,799 ('"799 patent"). Following stipulations dismissing all claims concerning the '400 patent and '799 patent, the court dismissed these cases from the consolidated action: Civil Action Nos. 14-cv-665, 14-cv-666, 14-cv-695, 14-cv-698, and 14-cv-845. 1 Pursuant to Federal Rule of Civil Procedure 52(a), having considered the entire record in this case and the applicable law, the court concludes that the asserted claims of the RE'186 patent are not invalid due to obviousness. -

Availability and Regulatory Status of Major Antiretroviral Drugs (September 2009)
Availability and regulatory status of major antiretroviral drugs (September 2009) Medium price per patient per year (in U$) Suppliers International Preferred 2008 Dosage Nonproprietary Strengths form Lower Upper Name (INN) (adults) Low income Middle Middle Originator Generics income income Aurobindo Pharma Ltd.; Cipla Ltd.; Eastern Surgical Company; Emcure; Hetero Drugs 317 a 370 Ltd.; Laboratorio Elea S.A.C.I.F.y A; Abacavir tablets 300 mg 350 a GlaxoSmithKline (286-370) (353-401) Laborotorios Filaxis Argentina; Laborotorios (313-374) Richmond S.A.C.I.F; Matrix Laboratories Ltd.; Ranbaxy Ltd; Strides Arcolab Ltd. 238 a Matrix, Ranbaxy, Hetero; Cipla; Aurobindo; Tenofovir tablets 300 mg 166 a 207 a Gilead Sciences (238-420) Emcure; Aspen; Strides Arcolab Ltd. (151-207) (177-256) Apotex Mexico (Protein, S.A. de C.V.); Aspen Pharmacare Ltd.; Aurobindo Pharma Ltd.; Cipla Ltd.; Cristalia, productos quimicos 212 a 462 Bristol-Myers farmaceuticos Ltda.; Emcure; Hetero Drugs 100 mg 251 a (187-235) (242-1100) Squibb Ltd.; Laboratorio Dosa S.A; Laborotorios (187-310) Filaxis Argentina; Laborotorios Richmond buffered S.A.C.I.F; Ranbaxy Ltd.; Zhejiang Huahai tablets Pharmaceutical Co., Ltd. 208 b Bristol-Myers Aspen Pharmacare Ltd.; Aurobindo Pharma 150 mg Didanosine (172-244) Squibb Ltd.; Cipla Ltd. Aurobindo Pharma Ltd; Barr Laboratories, Inc; 242 a 238 a 220 Bristol-Myers 200 mg Cipla Ltd.; Laborotorios Richmond S.A.C.I.F; (197-310) (233-794) (217-235) Squibb Macleods Pharmaceuticals Ltd.; Ranbaxy Ltd.; 242 a 238 a 220 Bristol-Myers 200 mg Aurobindo Pharma Ltd; Barr Laboratories, Inc; (197-310) (233-794) (217-235) Squibb EC 223 799 997 Bristol-Myers Aurobindo Pharma Ltd.; Barr Laboratories, 250 mg capsules (214-223) (675-874) (613-997) Squibb Inc; Cipla Ltd.; Ranbaxy Ltd.; 288 1267 a 1269 Bristol-Myers Aurobindo Pharma Ltd.; Barr Laboratories; 400 mg (279-288) (507-1302) (1129-1386) Squibb Hetero Drugs Ltd.; Cipla Ltd.; Ranbaxy Ltd. -

Company City State 3 W LLC MYRTLE BEACH SC 4 Consulting
Company City State 3 W LLC MYRTLE BEACH SC 4 Consulting Inc DALLAS TX 4S Technologies LLC PALATINE IL 4-Serv Solutions Inc ROCHESTER MN 4-Serve Solutions Inc Wixom MI 6COM Inc Piscataway NJ 9to9 Software Solutions LLC EAST BERLIN CT A&T of Tampa, LLC TAMPA FL AAA - The Auto Club Group TAMPA FL AACSB International TAMPA FL ABAL Technologies TRENTON NJ ABB Inc. WINDSOR CT ABM Industrial and Manufacturing Sarasota FL Absolutdata Technologies Inc. ALAMEDA CA ABSOMAX INC Novi MI Acadia Technologies, Inc. DULUTH GA Accesso LLC LAKE MARY FL Accuity, Inc TEMPLE TERRACE FL Aclara Technologies LLC SAINT LOUIS MO Acosta Sales and Marketing TEMPLE TERRACE FL Acro Staffing, Inc. LIVONIA MI ACS System Associates, Inc. MOUNT VERNON NY Actiontec Electronics SUNNYVALE CA Active8 BROOKHAVEN GA Acts Consulting Inc dba Acts 360 PLANT CITY FL Acuty LLC FLUSHING NY AcuVeterans, Inc LARGO FL ADAM INFORMATION TECHNOLOGIES LLC RICHARDSON TX Adam Infotech Madison WI Addepar Inc. SALT LAKE CITY UT Admaru Network LLC FORT LEE NJ ADT Security Services BOCA RATON FL Advanced Aerodynamics,LLC HALLANDALE BEACH FL Advanced Impact Technologies LARGO FL Advanced Structural Technologies, Inc. METAIRIE LA Adventist Health System ALTAMONTE SPRINGS FL Adventist Health System TAMPA FL Advocates for World Health Tampa FL AECOM Technical Services, Inc. TAMPA FL Aerotek Memphis TN Aerotek SCHAUMBURG IL Aflac Inc. NORTHGLENN CO Agile SDE, LLC MELBOURNE FL AGORA SAINT PETERSBURG FL Agora Edge ST PETERSBURG FL Agreeya solutions FOLSOM CA Agreeya Solutions LACEY WA AHS Information Services Altamonte Springs FL Aidble Inc Cheyenne WY Aids Healthcare Foundation LOS ANGELES CA Aimtron Corporation PALATINE IL AirSage, Inc. -

Cipla Limited
Cipla Limited Registered Office: Cipla House, Peninsula Business Park, Ganpatrao Kadam Marg, Lower Parel, Mumbai – 400 013 Phone: (9122) 2482 6000, Fax: (9122) 2482 6893, Email: [email protected], Website: www.cipla.com Corporate Identity Number: L24239MH1935PLC002380 Annexure to the Board’s Report Particulars of employee remuneration for the financial year ended 31st March, 2019 As required under section 197(12) of the Companies Act, 2013, read with rule 5(2) and (3) of the Companies (Appointment and Remuneration of Managerial Personnel) Rules, 2014. Employed throughout the year Name Designation Qualification Experience Age Date of Last employment Remuneration (in years) (in years) Employment (Rs.) Abhay Kumar Chief Talent Officer Master of Arts / 17 53 3/10/2016 Piramal Pharma 15,034,298.00 Srivastava Master of Personal Solutions Management Ademola Olukayode Head - Quality Doctorate / MPH / B. 17 48 20/6/2018 US FOOD AND DRUG 17,982,961.00 Daramola Compliance & Tech. ADMINISTRATION Sustainability (US FDA) Ajay Luharuka Head Finance - IPD, B.com,MMS,CFA 23 46 11/7/1996 NIIT Limited 11,922,994.00 API, Specialty & Global Respi Aliakbar Rangwala Senior Business Head M. Sc. / B. Sc. 19 42 19/1/2009 NA 10,677,779.00 - Chronic & Emerging - India Business Alpana Vartak Head - Talent MBA (HR) / B. Sc. 15 41 8/1/2018 Coco - Cola 10,312,782.00 Acquisition Company Anil Kartha Site Head - Bsc, Bpharm 28 56 27/5/1991 Vysali 12,525,338.00 Patalganga - Pharmaceuticals Formulations Anindya Kumar Shee Head - Organization B. Tech. / MBA 18 48 14/1/2016 Reliance Industries 11,084,298.00 Development Ltd. -

Package Leaflet: Information for the User Paroxetine Aurobindo 20 Mg
Package leaflet: Information for the user Paroxetine Aurobindo 20 mg film-coated tablets Paroxetine Aurobindo 30 mg film-coated tablets Paroxetine Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor,or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Paroxetine Aurobindo is and what it is used for 2. What you need to know before you take Paroxetine Aurobindo 3. How to take Paroxetine Aurobindo 4. Possible side effects 5. How to store Paroxetine Aurobindo 6. Contents of the pack and other information 7. 1. What Paroxetine Aurobindo is and what it is used for Paroxetine Aurobindo belongs to a group of medicines called Selective Serotonin Reuptake Inhibitors (SSRIs), which are antidepressants. Paroxetine Aurobindo is used in the treatment of: - Depression (major depressive episodes). - Obsessive Compulsive Disorder (compulsive thoughts and compulsive actions) (OCD). - Panic disorder with and without agoraphobia (e.g. fear of leaving the house, entering shops, or fear of public places). - Social phobia (overwhelming fear or avoidance of everyday social situations). - Generalized anxiety disorder (more permanent present fear, in which cronic nervous worring is prominent).