Chronic Hepatitis C Treatment Expansion: Generic Manufacturing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Responding to Mylan's Inadequate Tender Offer
Responding To Mylan’s Inadequate Tender Offer: Perrigo’s Board Recommends That You Reject the Offer and Do Not Tender September 2015 Important Information Forward Looking Statements Certain statements in this presentation are forward-looking statements. These statements relate to future events or the Company’s future financial performance and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control, including future actions that may be taken by Mylan in furtherance of its unsolicited offer. These and other important factors, including those discussed under “Risk Factors” in the Perrigo Company’s Form 10-K for the year ended June 27, 2015, as well as the Company’s subsequent filings with the Securities and Exchange -

J. SCOTT MCBRIDE PARTNER ━ 54 West Hubbard Street, Chicago, IL 60654 | 312.494.4436 | [email protected]
J. SCOTT MCBRIDE PARTNER ━ 54 West Hubbard Street, Chicago, IL 60654 | 312.494.4436 | [email protected] EDUCATION & HONORS The University of Chicago Law School, 2002, J.D., with High Honors Order of the Coif 1st Place, Hinton Moot Court The University of Chicago Law Review Northwestern University, 1994, M.S. Ed., Earned Illinois Secondary Teaching Certificate for Chemistry, Physics and German Dartmouth College, 1992, A.B., summa cum laude, High Honors in Chemistry Phi Beta Kappa Rufus Choate Scholar German Academic Exchange Service Scholarship Chandler T. White Chemistry Research Prize CLERKSHIPS Honorable Richard D. Cudahy, United States Court of Appeals for the Seventh Circuit, 2002-2003 ADMISSIONS Illinois INTELLECTUAL PROPERTY LITIGATION ViiV Healthcare v. Gilead Sciences (D. Del.) Lead trial counsel for Gilead in patent infringement case related to Gilead's Biktarvy® HIV treatment. Case pending. www.bartlit-beck.com CHICAGO 312.494.4400 DENVER 303.592.3100 J. SCOTT MCBRIDE Adverio Pharma GmbH v. Alembic Pharmaceuticals, Ltd. et al. (D. Del.) Lead trial counsel for Adverio and Bayer in Hatch-Waxman litigation relating to Bayer's Adempas® (riociguat) treatment for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension. Gilead Sciences v. Mylan Pharmaceuticals (D. Del.) Lead trial counsel for Gilead in Hatch-Waxman litigation relating to Gilead's Tybost® (cobicistat) product. Case settled favorably. UroPep v. Eli Lilly (E.D. Tex.) Lead trial counsel (with my partners John Hughes) for UroPep in patent infringement action related to Lilly's Cialis product. Trial verdict for UroPep with damages of $20 million; affirmed on appeal. Regents of the Univ. -

Pfizer and Mylan Will Own 57% and 43% of the New Company (“Newco”), Respectively
DEAL PROFILE PFIZER | MYLAN VALUES 57% 43% $12.0bn PFIZER SHARE OF NEWCO MYLAN SHARE OF NEWCO NEW DEBT RAISED Pfizer Inc. (NYSE:PFE) Pfizer Inc. (“Pfizer”) is a pharmaceutical company that develops, manufactures, and sells products in internal medicine, vaccines, oncology, inflammation & immunology, and rare diseases. Upjohn, a subsidiary of Pfizer, is composed of Pfizer’s oral solid generics and off-patent drugs franchise, such as Viagra, Lipitor, and Xanax. Upjohn has a strong focus in emerging markets, and is headquartered in Shanghai, China. TEV: $265.4bn LTM EBITDA: $22.5bn LTM Revenue: $55.7bn Mylan N.V. (NASDAQ:MYL) Mylan N.V. (“Mylan”) is a pharmaceutical company that develops, manufactures, and commercializes generic, branded-generic, brand-name, and over-the-counter products. The company focuses in the following therapeutic areas: cardiovascular, CNS and anesthesia, dermatology, diabetes and metabolism, gastroenterology, immunology, infectious disease, oncology, respiratory and allergy, and women’s health. Mylan was founded in 1961 and is headquartered in Canonsburg, PA. TEV: $23.9bn LTM EBITDA: $3.5bn LTM Revenue: $11.3bn Bourne Partners provides strategic and financial advisory services to BOURNE clients throughout the business evolution life cycle. In order to provide PARTNERS the highest level of service, we routinely analyze relevant industry MARKET trends and transactions. These materials are available to our clients and partners and provide detailed insight into the pharma, pharma services, RESEARCH OTC, consumer health, and biotechnology sectors. On July 29, 2019, Pfizer announced the spin-off of its off-patent drug unit, Upjohn, in order to merge the unit with generic drug manufacturer, Mylan. -

Mylan Signs Agreement with Gilead to Enhance Access to Tenofovir Alafenamide (TAF)- Based HIV Treatments in Developing Countries
December 1, 2014 Mylan Signs Agreement with Gilead to Enhance Access to Tenofovir Alafenamide (TAF)- based HIV Treatments in Developing Countries PITTSBURGH and HYDERABAD, India, Dec. 1, 2014 /PRNewswire/ -- Mylan Inc. (Nasdaq: MYL) today announced that its subsidiary Mylan Laboratories Limited has entered into an agreement with Gilead Sciences, Inc. under which Mylan has licensed the non-exclusive rights to manufacture and distribute Tenofovir Alafenamide (TAF) as both a single agent product and in combination with other drugs. Tenofovir Alafenamide (TAF) is an investigational antiretroviral drug for the treatment of HIV-1 infection. The license being granted to Mylan extends to 112 countries, which together account for more than 30 million people living with HIV, representing 84% of those infected globally.1 Mylan CEO Heather Bresch said, "Mylan's mission is to provide the world's 7 billion people access to high quality medicines and set new standards in health care. By working with partners like Gilead to help ensure access to innovative new products such as Tenofovir Alafenamide (TAF) in the countries hardest hit by this disease, we can help stem the tide of HIV/AIDS around the world." As part of the licensing agreement, on U.S. Food and Drug Administration (FDA) approval, Mylan will receive a technology transfer from Gilead, enabling the company to manufacture low-cost versions of Tenofovir Alafenamide (TAF), if approved as a single agent or in approved combinations containing Tenofovir Alafenamide (TAF) for developing markets. Phase III trials by Gilead Sciences met their primary objective supporting the potential for Tenofovir Alafenamide (TAF) to provide a new treatment option for individuals living with HIV. -

Inner 24 India Pharma & Healthcare Fund Low
Tata India Pharma & Healthcare Fund (An open ended equity scheme investing in Pharma and Healthcare Services Sector) As on 30th June 2020 PORTFOLIO INVESTMENT STYLE Company name No. of Market Value % of Company name No. of Market Value % of Primarily focuses on investment in at least 80% of its net Shares Rs. Lakhs Assets Shares Rs. Lakhs Assets assets in equity/equity related instruments of the Equity & Equity Related Total 23733.48 98.49 companies in the Pharma & Healthcare sectors in India. Healthcare Services Other Equities^ 380.78 1.58 INVESTMENT OBJECTIVE Narayana Hrudayalaya Ltd. 382720 1025.69 4.26 Repo 871.61 3.62 The investment objective of the scheme is to seek long Healthcare Global Enterprises Ltd. term capital appreciation by investing atleast 80% of its 410000 503.48 2.09 Portfolio Total 24605.09 102.11 net assets in equity/equity related instruments of the Apollo Hospitals Enterprise Ltd. 32500 438.70 1.82 Net Current Liabilities -507.01 -2.11 companies in the pharma & healthcare sectors in Pharmaceuticals India.However, there is no assurance or guarantee that Net Assets 24098.08 100.00 the investment objective of the Scheme will be Sun Pharmaceutical Industries Ltd. 870100 4115.14 17.08 achieved.The Scheme does not assure or guarantee any Dr Reddys Laboratories Ltd. 98000 3866.05 16.04 returns. Divi Laboratories Ltd. 102500 2335.87 9.69 DATE OF ALLOTMENT Lupin Ltd. 223000 2033.31 8.44 December 28, 2015 Ipca Laboratories Ltd. 95000 1590.68 6.60 Cipla Ltd. 230000 1472.58 6.11 FUND MANAGER Aurobindo Pharma Ltd. -

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

1976 3M Medical Solutions Division 2019 Electrocore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc
CURRENT SUSTAINING MEMBER COMPANIES MEMBER FOR OVER: 10 Years 25 Years 50 Years Member Since (alphabetical order) 1976 3M Medical Solutions Division 2019 electroCore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc. 2010 Endo Pharmaceuticals 2018 Organogenesis 2013 AbbVie Inc. 2017 Exelixis 2004 Otsuka America Pharmaceutical, Inc. 2021 Adaptive Biotechnologies 2016 Express Scripts Federal Pharmacy 2018 Pacira BioSciences, Inc. 2017 ACADIA Pharmaceuticals, Inc. 2010 Federal Practitioner 2018 Paratek Pharmaceuticals 2020 AcelRx Pharmaceuticals, Inc. 2018 Foundation Medicine, Inc. 1990 Pfizer Pharmaceuticals 2020 Acorda Therapeutics 2021 Frontier Technology Inc. (FTI) 2017 Pharmacyclics, LLC 2019 Aimmune 2020 Fresenius Medical Care North America 2020 RedHill BioPharma 2003 Alcon Laboratories, Inc. 1989 Genentech Inc. 2019 Red One Medical 2019 Alexion Pharmaceuticals, Inc. 2006 Gilead Sciences 2020 Regeneron 2017 Alkermes, Inc. 1983 GLAXOSMITHKLINE 2009 Regenesis Biomedical, Inc. 2019 Alnylam Pharmaceuticals 2013 Golden State Medical Supply, Inc. 2011 Remund Group, LLC 2019 Altarum Institute 2020 GRAIL 2018 Rigel Pharmaceuticals 2020 Amarin Corporation 2019 Greenwich Biosciences 2000 Sanofi 1994 AmerisourceBergen 2013 Gulf Coast Pharmaceuticals Plus, LLC 2020 Seattle Genetics 1992 Amgen 2008 Heritage Health Solutions, Inc. 2004 Siemens Medical Solutions 2020 Amneal Pharmaceutical 2017 Hill-Rom Company 2019 SK Life Science, Inc. 2019 Aptive Resources LLC 2020 Immunomedics 2002 Smith & Nephew, Inc. 2020 The Arbinger Institute 2019 ImmunoVation, LLC 2019 Sobi Inc. 2011 Arbor Pharmaceuticals, LLC 2019 Incyte Corporation 2013 Stryker Orthopaedics 2010 Argentum Medical, LLC 2019 Indivior 2018 Sun Pharmaceutical 2019 ASM Research, LLC 2015 Intercept Pharmaceuticals 1999 Sunovion Pharmaceuticals, Inc. 1986 Astellas Pharma US, Inc. 2019 Ipsen Biopharmaceuticals, Inc. 2016 Taiho Oncology, Inc. 1995 AstraZeneca 2018 IT Cadre 2015 Takeda Oncology 2020 Baudax Bio, Inc. -

Annual-Report-2018-1
Index Corporate Overview 2-33 About NATCO Pharma 2 Key milestones 6 Business highlights FY 2018-19 8 Key performance indicators 10 Megatrends 12 Management message 14 Building businesses in diverse markets 18 Leveraging synergies through diversity 20 Advancing R&D diversification 22 Board of Directors and Key 24 Management Team Risk framework and mitigation 26 strategy Corporate social responsibility (CSR) 28 Environment, health & safety (EHS) 32 Key numbers of FY 2018-19 on consolidated basis Statutory Reports 34-98 Total Revenue PAT Management Discussion and Analysis 34 Board's Report 43 ` 22,247 million ` 6,444 million Corporate Governance Report 71 Business Responsibility Report 90 EBITDA Basic EPS ` 9,250 million ` 34.98 Financial Statements 99-192 Disclaimer Standalone Financials 99 In this Annual Report, we have disclosed forward-looking information to enable investors to comprehend our prospects and take investment decisions. This report and Consolidated Financials 147 other statements - written and oral - that we periodically make contain forward-looking statements that set out anticipated results based on the management’s plans and assumptions. We have tried, wherever possible, to identify such statements by using words such as ‘anticipate’, ‘estimate’, ‘expects’, ‘projects’, ‘intends’, ‘plans’, ‘believes’, and Notice 193 words of similar substance in connection with any discussion of future performance. We cannot guarantee that these forward-looking statements will be realised, although we believe we have been prudent in our assumptions. The achievements of results are subject to risks, uncertainties and even inaccurate assumptions. Should known or unknown risks or uncertainties materialise, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. -

COVID-19 Pharmacotherapy MATTHEW F
COVID-19 Pharmacotherapy MATTHEW F. DERAEDT, PHARM.D., B.C.P.S. ANMC EMERGENCY DEPARTMENT PHARMACIST LT, USPHS Disclosure •No conflicts of interest to disclose •Recommendations to follow primarily from Alaska Native Medical Center’s Policy and Procedures •Clinical questions regarding specific patients should be deferred to ANMC Infectious Disease Department •Rapidly evolving FDA EUA and approved indications Objectives •Understanding inclusion and exclusion criteria for COVID-19 pharmacotherapies •Mechanism of action (MOA), pharmacokinetics, and pharmacodynamics for various therapies •Monitoring parameters for pharmacotherapies •Regulatory requirements for emergency use authorization therapies Remdesivir (Veklury®) •Adenosine nucleotide analog prodrug that is metabolized intracellularly to a nucleoside monophosphate intermediate •The nucleoside monophosphate is phosphorylated to active compound nucleoside triphosphate metabolite •The remdesivir- triphosphate (RDV TP) competes with ATP for incorporation into nascent RNA chains by SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) •Inhibits RNA chain termination Adamsick ML, et al. JASN. 2020:31(7):1384 - 1386 Remdesivir [package insert]. Foster City, CA. Gilead Sciences Inc. 2020. Road To Approval •FDA released on Emergency Use Authorization (EUA) May 1, 2020 •FDA approval on October 22, 2020 for adult patients and pediatric patients 12 years of age or older weighing at least 88 lbs (40kg) for the treatment of hospitalized COVID-19 patients •No longer need to follow EUA requirements for the approved patient population •Three Clinical Trials 1. NIAID ACTT-1: Mild/moderate and Severe COVID-19 2. GS-US-540-5774: Moderate COVID-19 3. GS-US-540-5773: Severe COVID-19 Remdesivir [package insert]. Foster City, CA. Gilead Sciences Inc. 2020. Spinner CD, et al. -

Pharmaceutical Cluster in Andhra Pradesh
Pharmaceutical Cluster in Andhra Pradesh Microeconomics of Competitiveness Final Project Harvard Business School Helene Herve | Lhakpa Bhuti | Saurabh Agarwal | Sonny Kushwaha | Akbar Causer May 2013 Table of Contents 1 Executive Summary ............................................................................................................................ 3 2 Introduction to India ........................................................................................................................... 4 2.1 History and Political Climate ....................................................................................................... 5 2.2 Competitive Positioning of India ................................................................................................. 6 2.2.1 Endowments .......................................................................................................................... 6 2.2.2 Economic Performance To-Date and Macroeconomic Policy .............................................. 7 2.2.3 Summary of Export Clusters ................................................................................................. 9 2.2.4 Social Infrastructure and Political Institutions .................................................................... 10 2.2.5 India Diamond .................................................................................................................... 11 3 Andhra Pradesh ................................................................................................................................ -
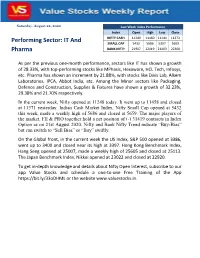
Nifty Poised to Open Gap up on Strong Payroll Data
Saturday, August 22, 2020 Last Week Index Performance Index Open High Low Close NIFTY CASH 11249 11460 11144 11372 Performing Sector: IT And SMALL CAP 5432 5686 5357 5659 Pharma BANK NIFTY 21907 22419 21403 22300 As per the previous one-month performance, sectors like IT has shown a growth of 28.33%, with top-performing stocks like MPhasis, Hexaware, HCL Tech, Infosys, etc. Pharma has shown an increment by 21.88%, with stocks like Divis Lab, Alkem Laboratories, IPCA, Abbot India, etc. Among the Minor sectors like Packaging, Defence and Construction, Supplies & Fixtures have shown a growth of 32.23%, 29.38% and 21.70% respectively. In the current week, Nifty opened at 11248 today. It went up to 11458 and closed at 11371 yesterday. Indian Cash Market Index, Nifty Small Cap opened at 5432 this week, made a weekly high of 5686 and closed at 5659. The major players of the market, FII & PRO together hold a net position of (-) 31419 contracts in Index Option as on 21st August 2020. Nifty and Bank Nifty Trend indicate “Buy-Bias” but can switch to “Sell Bias” or “Buy” swiftly. On the Global front, in the current week the US Index, S&P 500 opened at 3386, went up to 3400 and closed near its high at 3397. Hong Kong Benchmark Index, Hang Seng opened at 25007, made a weekly high of 25605 and closed at 25113. The Japan Benchmark Index, Nikkei opened at 23022 and closed at 22920. To get in-depth knowledge and details about Nifty Open Interest, subscribe to our app Value Stocks and schedule a one-to-one Free Training of the App https://bit.ly/33oDHML or the website www.valuestocks.in.