Lateral Exuvial Splits in Leaf-Mining Larvae of Pachyschelus (Coleoptera: Buprestidae) and Cameraria (Lepidoptera: Gracillariidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera on the Introduced Robinia Pseudoacacia in Slovakia, Central Europe
Check List 8(4): 709–711, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Lepidoptera on the introduced Robinia pseudoacacia in PECIES S OF ISTS L Slovakia, Central Europe Miroslav Kulfan E-mail: [email protected] Comenius University, Faculty of Natural Sciences, Department of Ecology, Mlynská dolina B-1, SK-84215 Bratislava, Slovakia. Abstract: Robinia pseudoacacia A current checklist of Lepidoptera that utilize as a hostplant in Slovakia (Central Europe) faunalis provided. community. The inventory Two monophagous is based on species, a bibliographic the leaf reviewminers andMacrosaccus new unreported robiniella data and from Parectopa southwest robiniella Slovakia., and Thethe polyphagouslist includes 35pest Lepidoptera Hyphantria species cunea belonging to 10 families. Most species are polyphagous and belong to Euro-Siberian have subsequently been introduced to Slovakia. Introduction E. The area is a polygon enclosed by the towns of Bratislava, Robinia pseudoacacia a widespread species in its native habitat in southeastern North America. It was L.introduced (black locust, to orEurope false acacia),in 1601 is Komárno, Veľký Krtíš and Myjava. Ten plots were located in the southern part of the study area. Most were located in theThe remnant trophic ofgroups the original of the floodplain Lepidoptera forests larvae that found were (Chapman 1935). The first mention of planting the species distributed along the Danube and Morava rivers. (Keresztesiin Slovakia dates 1965). from Today, 1750, itwhen is widespread black locust wasthroughout planted (1986). The zoogeographical distribution of the species western,around the central, fortress eastern in Komárno and southern in southern Europe, Slovakia where followswere defined the arrangement following the give system by Reiprichof Brown (2001). -

Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015)
Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015) By Richard Henderson Research Ecologist, WI DNR Bureau of Science Services Summary This is a preliminary list of insects that are either well known, or likely, to be closely associated with Wisconsin’s original native prairie. These species are mostly dependent upon remnants of original prairie, or plantings/restorations of prairie where their hosts have been re-established (see discussion below), and thus are rarely found outside of these settings. The list also includes some species tied to native ecosystems that grade into prairie, such as savannas, sand barrens, fens, sedge meadow, and shallow marsh. The list is annotated with known host(s) of each insect, and the likelihood of its presence in the state (see key at end of list for specifics). This working list is a byproduct of a prairie invertebrate study I coordinated from1995-2005 that covered 6 Midwestern states and included 14 cooperators. The project surveyed insects on prairie remnants and investigated the effects of fire on those insects. It was funded in part by a series of grants from the US Fish and Wildlife Service. So far, the list has 475 species. However, this is a partial list at best, representing approximately only ¼ of the prairie-specialist insects likely present in the region (see discussion below). Significant input to this list is needed, as there are major taxa groups missing or greatly under represented. Such absence is not necessarily due to few or no prairie-specialists in those groups, but due more to lack of knowledge about life histories (at least published knowledge), unsettled taxonomy, and lack of taxonomic specialists currently working in those groups. -
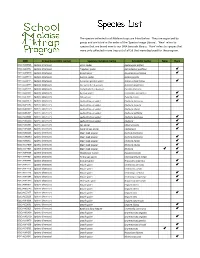
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

Coleoptera: Chrysomelidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 9-2-2011 Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) R. F. C. Naczi The New York Botanical Garden, [email protected] C. L. Staines National Museum of Natural History, Smithsonian Institution, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Naczi, R. F. C. and Staines, C. L., "Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae)" (2011). Insecta Mundi. 702. https://digitalcommons.unl.edu/insectamundi/702 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0190 Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) R. F. C. Naczi The New York Botanical Garden 2900 Southern Blvd. Bronx, NY 10458-5126, U.S.A. C. L. Staines Department of Entomology, MRC 187 National Museum of Natural History, Smithsonian Institution Washington, DC 20013-7012, U.S.A. Date of Issue: September 2, 2011 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL R. F. C. Naczi and C. L. Staines Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) Insecta Mundi 0190: 1-6 Published in 2011 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 U. S. A. http://www.centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. -

Annual Report 2011
Annual Report 2011 © RMCA www.africamuseum.be Foreword 2 Foreword The Royal Museum for Central Africa (RMCA) pub- and culture exhibition was extended, while RMCA lishes a beautiful and richly illustrated annual collection pieces were admired in more than 20 report in book form every two years. In intervening major exhibitions held in different parts of the years – such as 2011 – we publish a digital edition globe. Nearly 30,000 children attended our edu- that is available on our website, and for which a cational workshops or school activities, while our hard copy can be produced on demand. Despite colla boration with African communities became its size, the report is not exhaustive. Rather, it more streamlined. We felt a pang of regret at the seeks to provide the most varied overview pos- departure of ‘our’ elephants in 2011. After grac- sible of our many museum-related, educational, ing our museum’s entrance for three years, the scientific, and other activities on the national and 9 pachyderms that formed the work created by international scene. The long governmental crisis South African artist Andries Botha, You can buy my of 2011 notwithstanding, RMCA was highly pro- heart and my soul, left Tervuren Park for good. ductive and remains one of the most important Africa-focused research institutions, particularly 2011 was also a fruitful year in terms of scientific for Central Africa. research. To highlight the multidisciplinary nature that is the strength of our institution, we organ- As with the previous year, the renovation was one ized ‘Science Days’ for the first time. -

Systematics, Phylogeny and Biology of a New Genus of Lithocolletinae (Lepidoptera: Gracillariidae) Associated with Cistaceae
Zootaxa 3741 (2): 201–227 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3741.2.1 http://zoobank.org/urn:lsid:zoobank.org:pub:E37C82A2-27DA-42DE-A298-838578F6B179 Systematics, phylogeny and biology of a new genus of Lithocolletinae (Lepidoptera: Gracillariidae) associated with Cistaceae JURATE DE PRINS1,4, DONALD R. DAVIS2, ELIANE DE CONINCK1, JAE-CHEON SOHN2 & PAOLO TRIBERTI3 1Royal Museum for Central Africa, Tervuren, Belgium 2National Museum of Natural History, Smithsonian Institution, USA 3Museo di Storia Naturale, Verona, Italy 4Corresponding author. E-mail: [email protected] Abstract The gracillariid genus Triberta gen. nov. (Lepidoptera: Gracillariidae: Lithocolletinae Stainton, 1854) is described to ac- commodate two species formerly assigned to the genus Phyllonorycter Hübner, 1822: Triberta helianthemella (Herrich- Schäffer, 1861) comb. nov. and T. cistifoliella (Groschke, 1944) comb. nov. Triberta cistifoliella bona sp. is restored from synonymy based on morphological characters. The new genus is biologically associated with the plant family Cistaceae of the order Malvales and is endemic to the Palaearctics. Our molecular analysis of eleven nuclear genes failed to unambiguously place Triberta in the lithocolletine phylogeny, but revealed that this genus is distinct from either clade Phyllonorycter + Cremastobombycia and Cameraria. The distinctiveness of Triberta is also supported by inferred -

Section IV – Guideline for the Texas Priority Species List
Section IV – Guideline for the Texas Priority Species List Associated Tables The Texas Priority Species List……………..733 Introduction For many years the management and conservation of wildlife species has focused on the individual animal or population of interest. Many times, directing research and conservation plans toward individual species also benefits incidental species; sometimes entire ecosystems. Unfortunately, there are times when highly focused research and conservation of particular species can also harm peripheral species and their habitats. Management that is focused on entire habitats or communities would decrease the possibility of harming those incidental species or their habitats. A holistic management approach would potentially allow species within a community to take care of themselves (Savory 1988); however, the study of particular species of concern is still necessary due to the smaller scale at which individuals are studied. Until we understand all of the parts that make up the whole can we then focus more on the habitat management approach to conservation. Species Conservation In terms of species diversity, Texas is considered the second most diverse state in the Union. Texas has the highest number of bird and reptile taxon and is second in number of plants and mammals in the United States (NatureServe 2002). There have been over 600 species of bird that have been identified within the borders of Texas and 184 known species of mammal, including marine species that inhabit Texas’ coastal waters (Schmidly 2004). It is estimated that approximately 29,000 species of insect in Texas take up residence in every conceivable habitat, including rocky outcroppings, pitcher plant bogs, and on individual species of plants (Riley in publication). -

Phylogeographic Pattern of the Plane Leaf Miner, Phyllonorycter Platani (STAUDINGER, 1870) (Lepidoptera: Gracillariidae) in Europe Viktória Tóth and Ferenc Lakatos*
Tóth and Lakatos BMC Evolutionary Biology (2018) 18:135 https://doi.org/10.1186/s12862-018-1240-z RESEARCH ARTICLE Open Access Phylogeographic pattern of the plane leaf miner, Phyllonorycter platani (STAUDINGER, 1870) (Lepidoptera: Gracillariidae) in Europe Viktória Tóth and Ferenc Lakatos* Abstract Background: The plane leaf miner, Phyllonorycter platani is a widely distributed insect species on plane trees and has a well-documented colonisation history in Europe over the last century. However, phylogeographic data of the species are lacking. Results: We analysed 284 individuals from 38 populations across Europe, Asia, and North America. A 1242 bp fragment of the mitochondrial COI gene and an 893 bp fragment of the 28S rDNA has been Sanger sequenced. Twenty-four haplotypes were detected on the COI gene, and two alleles were identified on the 28S rDNA. We revealed two distinct clades for both markers reflecting the geographic origins, Asia and Europe. The genetic distance between the two main clades is 2.08% on the COI gene and 0.10% on the nuclear DNA. An overlapping zone of the two clades was found across Eastern Europe and the Anatolian Peninsula. We detected heterozygote individuals of the 28S rDNA gene in Moldavia, Ukraine and in the southern part of Turkey. These suggest that the two clades can hybridise. Furthermore, the presence of European type homozygote individuals has been confirmed in the southern part of Turkey as well. Conclusions: We have shown that both post-glacial recolonization and recent expansion events influenced the present genetic structure of P. platani. The genetic patterns revealed at least two refugia during the last ice age: one in the Balkan Peninsula and the other in the Caucasus region. -

Egg Laying Site Selection by a Host Plant Specialist Leaf Miner Moth at Two Intra-Plant Levels in the Northern Chilean Atacama Desert
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262014000300009 Egg laying site selection by a host plant specialist leaf miner moth at two intra-plant levels in the northern Chilean Atacama Desert José Storey-Palma1, Hugo A. Benítez2,3, Enrique A. Mundaca4 & Héctor A. Vargas1,5 1Departamento de Recursos Ambientales, Facultad de Ciencias Agronómicas, Universidad de Tarapacá, Casilla 6-D, Arica, Chile. [email protected] 2Faculty of Life Sciences, The University of Manchester, Manchester M13 9PT, United Kingdom. [email protected] 3Instituto de Alta Investigación, Universidad de Tarapacá, Casilla 7-D Arica, Chile 4Escuela de Agronomía, Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Casilla 7-D, Curicó, [email protected] 5Corresponding author: [email protected] ABSTRACT. Egg laying site selection by a host plant specialist leaf miner moth at two intra-plant levels in the northern Chilean Atacama Desert. The spatial distribution of the immature stages of the leaf miner Angelabella tecomae Vargas & Parra, 2005 was determined at two intra-plant levels (shoot and leaflet) on the shrub Tecoma fulva fulva (Cav.) D. Don (Bignoniaceae) in the Azapa valley, northern Chilean Atacama Desert. An aggregated spatial pattern was detected for all the immature stages along the shoot, with an age dependent relative position: eggs and first instar larvae were clumped at apex; second, third and fourth instar larvae were mostly found at intermediate positions; meanwhile the spinning larva and pupa were clumped at basis. This pattern suggests that the females select new, actively growing leaflets for egg laying. At the leaflet level, the immature stages were found more frequently at underside. -

Microlepidoptera.Hu Redigit: Fazekas Imre
Microlepidoptera.hu Redigit: Fazekas Imre 5 2012 Microlepidoptera.hu A magyar Microlepidoptera kutatások hírei Hungarian Microlepidoptera News A journal focussed on Hungarian Microlepidopterology Kiadó—Publisher: Regiograf Intézet – Regiograf Institute Szerkesztő – Editor: Fazekas Imre, e‐mail: [email protected] Társszerkesztők – Co‐editors: Pastorális Gábor, e‐mail: [email protected]; Szeőke Kálmán, e‐mail: [email protected] HU ISSN 2062–6738 Microlepidoptera.hu 5: 1–146. http://www.microlepidoptera.hu 2012.12.20. Tartalom – Contents Elterjedés, biológia, Magyarország – Distribution, biology, Hungary Buschmann F.: Kiegészítő adatok Magyarország Zygaenidae faunájához – Additional data Zygaenidae fauna of Hungary (Lepidoptera: Zygaenidae) ............................... 3–7 Buschmann F.: Két új Tineidae faj Magyarországról – Two new Tineidae from Hungary (Lepidoptera: Tineidae) ......................................................... 9–12 Buschmann F.: Új adatok az Asalebria geminella (Eversmann, 1844) magyarországi előfordulásához – New data Asalebria geminella (Eversmann, 1844) the occurrence of Hungary (Lepidoptera: Pyralidae, Phycitinae) .................................................................................................. 13–18 Fazekas I.: Adatok Magyarország Pterophoridae faunájának ismeretéhez (12.) Capperia, Gillmeria és Stenoptila fajok új adatai – Data to knowledge of Hungary Pterophoridae Fauna, No. 12. New occurrence of Capperia, Gillmeria and Stenoptilia species (Lepidoptera: Pterophoridae) ………………………. -

Ankara Ili Buprestidae (Insecta: Coleoptera) Familyasi Üzerinde Sistematik Araştirmalar
ANKARA İLİ BUPRESTIDAE (INSECTA: COLEOPTERA) FAMİLYASI ÜZERİNDE SİSTEMATİK ARAŞTIRMALAR SYSTEMATIC RESEARCHES ON THE FAMILY BUPRESTIDAE (INSECTA: COLEOPTERA) IN ANKARA PROVINCE ALİ KEMAL KIRÇAKCI DOÇ. DR. MAHMUT KABALAK Tez Danışmanı Hacettepe Üniversitesi Lisansüstü Eğitim-Öğretim ve Sınav Yönetmeliğinin Biyoloji (Uygulamalı Biyoloji) Anabilim Dalı için Öngördüğü YÜKSEK LİSANS TEZİ olarak hazırlanmıştır. 2020 ÖZET ANKARA İLİ BUPRESTIDAE (INSECTA: COLEOPTERA) FAMİLYASI ÜZERİNDE SİSTEMATİK ARAŞTIRMALAR Ali Kemal KIRÇAKCI Yüksek Lisans, Biyoloji Bölümü Tez Danışmanı: Doç. Dr. Mahmut KABALAK Haziran 2020, 235 sayfa Bu çalışmada Ankara ili Buprestidae familyası üzerinde araştırmalar yapılmıştır. Bu amaçla, Mayıs-Ekim 2018’de 33 gün ve Nisan-Ekim 2019’da 36 gün olmak üzere toplam 69 gün arazi çalışmaları yapılmıştır. Arazi çalışmaları sonucunda toplam 995 örnek toplanmıştır. 5 altfamilya ve 14 cinse ait toplam 44 tür teşhis edilmiştir. Buprestinae 3 cinse ait 19 türle en fazla türe sahip altfamilyadır. Agrilinae altfamilyasından 4 cinse ait 11 tür, Polycestinae altfamilyasından 3 cinse ait 7 tür, Chrysochroinae altfamilyasından 3 cinse ait 6 tür, Julodinae altfamilyasından ise 1 cinse ait 1 tür tespit edilmiştir. Tespit edilen tüm türlerin ayrıntılı lokalite kayıtları, sinonimleri, Türkiye ve Dünya yayılışları verilmiştir. Bu çalışmada tespit edilen türler sistematik ve taksonomik, faunistik, ekolojik ve zoocoğrafik özellikler bakımından değerlendirilmiş ve tartışılmıştır. Sistematik ve taksonomik değerlendirmelerde, 44 türün genel morfolojisi tanımlanmış ve fotoğraflanmıştır. Erkek örnekleri olan 36 türün erkek genital organları ayrıntılı bir şekilde tanımlanmış, dorsal ve lateral olarak fotoğraflanmış ve çizilmiştir. Erkek genital organları, tespit edilen türler literatür ile karşılaştırılmış ve tartışılmıştır. Mevcut literatür incelendiğinde Capnodis carbonaria, Klug, 1829, Perotis cuprata (Klug, 1829), i Acmaeodera (s. str) flavolineata Laporte & Gory, 1835, Acmaeoderella (Euacmaeoderella) villosula Steven, 1830, A.