The Egg-Cases Of.The Swell Shark, Cephal0scyllium Ventriosum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Elasmobranch Visual Ecology
City University of New York (CUNY) CUNY Academic Works Publications and Research Baruch College 2016 Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Elasmobranch Visual Ecology David F. Gruber CUNY Bernard M Baruch College Ellis R. Loew Cornell University Dimitri D. Deheyn University of California - San Diego Derya Akkaynak University of Haifa Jean P. Gaffney CUNY Bernard M Baruch College See next page for additional authors How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/bb_pubs/993 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Authors David F. Gruber, Ellis R. Loew, Dimitri D. Deheyn, Derya Akkaynak, Jean P. Gaffney, W. Leo Smith, Matthew P. Davis, Jennifer H. Stern, Vincent A. Pieribone, and John S. Sparks This article is available at CUNY Academic Works: https://academicworks.cuny.edu/bb_pubs/993 www.nature.com/scientificreports OPEN Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Received: 22 January 2016 Accepted: 05 April 2016 Elasmobranch Visual Ecology Published: 25 April 2016 David F. Gruber1,2,3, Ellis R. Loew4, Dimitri D. Deheyn5, Derya Akkaynak6,7, Jean P. Gaffney1, W. Leo Smith8, Matthew P. Davis9, Jennifer H. Stern8, Vincent A. Pieribone10 & John S. Sparks3,11 Biofluorescence has recently been found to be widespread in marine fishes, including sharks. Catsharks, such as the Swell Shark (Cephaloscyllium ventriosum) from the eastern Pacific and the Chain Catshark (Scyliorhinus retifer) from the western Atlantic, are known to exhibit bright green fluorescence. -

Database of Bibliography of Living/Fossil
www.shark-references.com Version 16.01.2018 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2017 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 DOI: 10.13140/RG.2.2.32409.72801 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 16.01.2018 Abstract: This paper contains a collection of 817 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2017. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2017, list of descriptions of extinct and extant species from 2017, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide data information on the geographic and depth distribution of newly described species, i.e. the type specimens from the years 1990 to 2017 in a hot spot analysis. New in this year's POTY is the subheader "biodiversity" comprising a complete list of all valid chimaeriform, selachian and batoid species, as well as a list of the top 20 most researched chondrichthyan species. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, possible errors cannot entirely be excluded. -

The Sharks of North America
THE SHARKS OF NORTH AMERICA JOSE I. CASTRO COLOR ILLUSTRATIONS BY DIANE ROME PEEBLES OXFORD UNIVERSITY PRESS CONTENTS Foreword, by Eugenie Clark v Mosaic gulper shark, Centrophorus tesselatus 79 Preface vii Little gulper shark, Centrophorus uyato 81 Acknowledgments ix Minigulper, Centrophorus sp. A 84 Slender gulper, Centrophorus sp. B 85 Introduction 3 Birdbeak dogfish, Deania calcea 86 How to use this book 3 Arrowhead dogfish, Deaniaprofundorum 89 Description of species accounts 3 Illustrations 6 Family Etmopteridae, The Black Dogfishes Glossary 7 and Lanternsharks 91 Bibliography 7 Black dogfish, Centroscyllium fabricii 93 The knowledge and study of sharks 7 Pacific black dogfish, Centroscyllium nigrum 96 The shark literature 8 Emerald or blurred lanternshark, Etmopterus bigelowi 98 Lined lanternshark, Etmopterus bullisi 101 Broadband lanternshark, Etmopterus gracilispinis 103 A KEY TO THE FAMILIES OF Caribbean lanternshark, Etmopterus hillianus 105 NORTH AMERICAN SHARKS 11 Great lanternshark, Etmopterusprinceps 107 Fringefin lanternshark, Etmopterus schultzi 110 SPECIES ACCOUNTS 19 Green lanternshark, Etmopterus virens 112 Family Chlamydoselachidae, The Frill Shark 21 Family Somniosidae, The Sleeper Sharks 115 Frill shark, Chlamydoselachus anguineus 22 Portuguese shark, Centroscymnus coelolepis 117 Roughskin dogfish, Centroscymnus owstoni 120 Family Hexanchidae, The Cowsharks 26 Velvet dogfish, Zameus squamulosus \T1 Sharpnose sevengill, or perlon shark, Heptranchias Greenland shark, Somniosus microcephalus 124 perlo 28 Pacific sleeper -

A Report Card for Australia's Sharks and Rays
Shark futures: A report card for Australia’s sharks and rays Colin Simpfendorfer, Andrew Chin, Cassandra Rigby, Samantha Sherman, William White March, 2019 FRDC Project No 2013/009 FRDC Project No 2013/009 xx 2017 © 2019 Fisheries Research and Development Corporation. All rights reserved. ISBN 978-0-9954471-2-7 Shark futures: a report card for Australia’s sharks and rays 2013/009 2019 Ownership of Intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Fisheries Research and Development Corporation and the Centre for Sustainable Tropical Fisheries and Aquaculture, James Cook University. This publication (and any information sourced from it) should be attributed to Simpfendorfer, C., Chin, A., Rigby, C., Sherman, S., White, W. (2017) Shark futures: a report card for Australia’s sharks and rays’, Centre for Sustainable Tropical Fisheries and Aquaculture, James Cook University, May. CC BY 3.0. Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from creativecommons.org/licenses/by/3.0/au/legalcode. Inquiries regarding the licence and any use of this document should be sent to: [email protected] Disclaimer The authors do not warrant that the information in this document is free from errors or omissions. -

Cephaloscyllium Laticeps
Thereproductivebiologyandmovement patternsofthedraughtboardshark, (Cephaloscylliumlaticeps ):implications forbycatchmanagement by Cynthia Andrea Awruch Submitted in fulfilment of requirements for the Degree of Doctor of Philosophy January 2007 Tasmanian Aquaculture andFisheries Institute School of Aquaculture University of Tasmania, Australia Draughtboard shark, Cephaloscyllium laticeps ii DECLARATIONS I hereby declare that this thesis is my own work except where due acknowledgement is given, andthat the material presentedhere has not been submitted at another university for the awardof any other degree diploma. This thesis my be made available for loan andlimitedcopying in accordance with the Copyright Act 1968 Cynthia Andrea Awruch January 2007 iii ABSTRACT The draughtboard shark ( Cephaloscyllium laticeps ) is the most common shark on temperate reefs in southeastern Australia. In order to implement adequate management plans its reproductive biology andmovement patterns were studied. Females developeda single external-type ovary with a maximum follicle diameter of 35 mm. Vitellogenesis commencedat 10 mm follicle diameter. The male reproductive tract consistedof pairedtestis with spermatocysts undergoing diametric development. The hormones testosterone, 17-β estradiol, progesterone and 11-ketotestosterone (males only) were examinedto determine their role in reproduction. Testosterone and estradiol showedmajor changes during follicle development. Estradiol increasedas the follicle developed before declining as the follicle reached maturity. Testosterone remained low during the first stages of follicular development and increased as the follicle reached maturity. Progesterone showed a peak just prior to ovulation. Testosterone was the only hormone that variedwith maturity in males andno levels of 11-ketotestosteorne were detected. Females were able to store sperm for at least 15 months andeggs were laidin pairs at monthly intervals. Juveniles hatchedafter 12 months. -
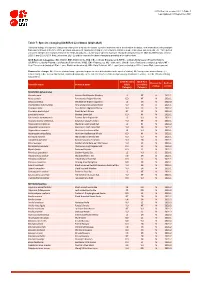
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-2: Table 7 Last Updated: 04 September 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

5Th Meeting of the Scientific Committee SC5-DW09 Rev1
5th Meeting of the Scientific Committee Shanghai, China, 23 - 28 September 2017 SC5-DW09_rev1 Ecosystem approach considerations: Deepwater chondrichthyans (sharks, rays and chimaeras) in the Western SPRFMO Area Clinton Duffy1, Shane Geange1 & Tiffany Bock2 1 Department of Conservation 2 Ministry for Primary Industries 1 23 Aug 2017 SC5-DW09_rev1 1. Purpose of paper This paper provides a characterisation of the catch of chondrichthyans in New Zealand bottom fisheries in the SPRFMO Area and information on potential risks to deepwater chondrichthyan species from SPRFMO bottom fishing. Chondrichthyans, particularly those which predominantly occur or complete most of their lifecycle below 200 m depth, are known to have life history characteristics which make them especially vulnerable to fishing pressure. 2. Background About half of chondrichthyans are considered deepwater species, of which around half are sharks (predominantly squaloid dogfishes, Order Squaliformes, and catsharks, Order Carcharhiniformes, Families Pentanchidae and Scyliorhinidae)), with the remainder being skates (predominantly Arhynchobatidae, Rajidae, and Anacanthobatidae), and holocephalans (Kyne & Simpfendorfer 2007). There are currently 177 species reported from the SPRFMO Area that are known to regularly occur below 200 m depth (Appendix 1). Chondrichthyans generally exhibit relatively slow growth rates, late age at maturity, low fecundity and low natural mortality. Knowledge of the growth and reproductive parameters of most deepwater species is generally poor or completely lacking. For the limited number of deepwater species for which sufficient life history data is available, their estimated intrinsic rebound potential values (i.e., ability of a species to recover from fishing pressure) fall at the lower end of the chondrichthyan productivity scale, and include the lowest levels observed (Kyne & Simpfendorfer 2007). -

Exploring Individual Attitudes Towards Sharks A
CALIFORNIA STATE UNIVERSITY NORTHRIDGE HUMAN-SHARK INTERACTIONS: EXPLORING INDIVIDUAL ATTITUDES TOWARDS SHARKS A thesis submitted in partial fulfillment of the requirements For the degree of Master of Arts in Anthropology By Katherine Palmer May 2015 The thesis of Katherine Palmer is approved: _____________________________________________ _______________ Dr. Kimberly Kirner Date _____________________________________________ _______________ Dr. Christina J. Campbell Date _____________________________________________ _______________ Dr. Sabina Magliocco, Chair Date California State University, Northridge ii Acknowledgments There are many people who made this thesis a reality. First and foremost, I would like to thank my father, Dr. A. Dean Palmer, for helping me decipher Smith’s S and for helping me make mathematical sense of all the data I collected. Thank you for providing invaluable assistance and advice with the statistical calculations as well as for the long hours spent editing my drafts. Thank you to my mother Cindy Palmer, who also spent long hours proofreading the first few drafts, and especially thank you for graciously putting up with all the different moods I went through in completing this research. Thank you to my advisor Dr. Magliocco, who believed in me and helped to shape this project from beginning to end. Thank you to Dr. Kirner for your invaluable insight into the world of cognitive anthropology. Thank you Dr. Campbell for finding worth in my research and for the helpful comments. Thank you Dr. Snead for making me feel like my research was important and for always taking the time to fill me in on interesting shark research going on around the world. I owe a debt to Nikki Cox for always keeping me on track and to Paige Plannette for patiently listening when I needed someone to just listen. -

Habitat Use and Foraging Ecology of a Batoid Community in Shark Bay, Western Australia Jeremy Vaudo Florida International University, [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 3-29-2011 Habitat Use and Foraging Ecology of a Batoid Community in Shark Bay, Western Australia Jeremy Vaudo Florida International University, [email protected] DOI: 10.25148/etd.FI11042706 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Recommended Citation Vaudo, Jeremy, "Habitat Use and Foraging Ecology of a Batoid Community in Shark Bay, Western Australia" (2011). FIU Electronic Theses and Dissertations. 367. https://digitalcommons.fiu.edu/etd/367 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida HABITAT USE AND FORAGING ECOLOGY OF A BATOID COMMUNITY IN SHARK BAY, WESTERN AUSTRALIA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Jeremy Vaudo 2011 iii To: Dean Kenneth Furton choose the name of dean of your college/school College of Arts and Sciences choose the name of your college/school This dissertation, written by Jeremy Vaudo, and entitled Habitat Use and Foraging Ecology of a Batoid Community in Shark Bay, Western Australia, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ John P. Berry _______________________________________ James W. Fourqurean _______________________________________ Philip K. -

Géominpal Belgica HERMAN Jacques Editor H
Géominpal Belgica Découvertes géologiques, minéralogiques et paléontologiques en Belgique 4 Observations concerning the Evolution and the Parasystematic of all the living and fossil Scyliorhiniformes and Carcharhiniformes and suggestions concerning the possible origins of the Batoidei. 2012 By Herman Jacques1 & Hilde Van Waes1 1Mail: [email protected] Extern side of one lower anterior tooth of Pteroscyllium sp. on its matrix. Uppermost phosphatic level (Cm1g) of the Trivières Chalk at Obourg, C.B.R. Quarry 4 (Province of Hainaut). Codification of the Belgian Geological Archives: 140 E 481. Collection Jacques Boel - Photo Pieter De Schutter HERMAN Jacques Editor H Géominpal Belgica Découvertes géologiques, minéralogiques et paléontologiques en Belgique. 4 Observations concerning the Evolution and the Parasystematic of all the living and fossil Scyliorhiniformes and Carcharhiniformes and suggestions concerning the possible origins of the Batoidei. By Herman Jacques & Hilde Van Waes December 2012 Editeur responsable: Docteur Jacques Herman. I.S.S.N. : 2033 - 6365 Beigemsesteenweg 319. 1852. Beigem (Grimbergen). Belgique – België – Belgien. G-Mail: [email protected] Web Site: www.geominpal.be -1- Dedication This work is dedicated to: Dr. Prof. Max Poll U.L.B. (Brussels, Belgium), whom initiated his student to the arcanes of the Zoological Systematic Respectfully, at Beigem 24 December 2012 Jacques Herman -2- Plan of this Publication 1.Summary – Résumé – Samenvatting– Kurzfassung: p. 6 2.Introduction: p. 7 3.Introduction: p. 7 4.Introductie: p. 8 5.The former Order Scyliorhiniformes and the Scyliorhinomorphii nov. Sup. Ord. : p. 8 5.1. The living Scyliorhinidae: p. 8 5.2. Taxa mentioned: p. 9 5.3. Size of the specimens: p. -

Species Delineation in the Southern African Endemic Catshark Genus Haploblepharus
Species delineation in the southern African endemic catshark genus Haploblepharus by Michaela van Staden Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in the Faculty of Natural Science at Stellenbosch University Supervisor: Dr A.E. Bester-van der Merwe Co-supervisor: Dr C. Rhode Department of Genetics December 2018 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Date: December 2018 Copyright © 2018 Stellenbosch University All rights reserved i | Page Stellenbosch University https://scholar.sun.ac.za Summary Accurate species identification is paramount for the effective implementation of conservation and management plans. Species identification in the genus Haploblepharus has historically been problematic due to the high degree of morphological conservatism between congeners, further complicated by the possibility of interspecific hybridisation. The research presented in this thesis addresses crucial knowledge gaps on species delineation in southern African endemic scyliorhinids by developing and applying molecular markers to assess species divergence in a morphologically conserved and threatened genus. Firstly, this study investigated the apparent lack of mitochondrial DNA sequence divergence previously reported among Haploblepharus species, using newly assembled mitochondrial genomes for Haploblepharus edwardsii, Haploblepharus pictus, Halaelurus natalensis and Poroderma pantherinum. -

Movements Patterns and Population Dynamics Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) M O V E M E NT PA T T E RNS A ND POPU L A T I O N D Y N A M I CS O F F O UR C A TSH AR KS E ND E M I C T O SO U T H A F RI C A Submitted in fulfilment of the requirements for the degree of M AST E R O F SC I E N C E at R H O D ES UNI V E RSI T Y by JESSI C A ESC O B A R-PO RR AS December 2009 Escobar-Porras, J. 2009 A BST R A C T Sharks are particularly vulnerable to over-exploitation. Although catsharks are an important component of the near-shore marine biodiversity in South Africa and most of the species are endemic, little is known about their movement patterns, home range and population size. With an increasing number of recreational fishers this information is crucial for their conservation. The aims of this study were threefold. Firstly, to identify and analyze existing data sources on movement patterns and population dynamics for four catshark species: pyjama (Poroderma africanum), leopard (P. pantherinum), puffadder (Haploblepharus edwarsii) and brown (H. fuscus). This highlighted a number of shortcomings with existing data sets, largely because these studies had diverse objectives and were not aimed solely at catsharks. Secondly, a dedicated study was carried out for a limited area, testing a number of methods for data collection, and where appropriate the data was analyzed to determine movement patterns and population numbers.