Vetscan Phenobarbital Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Thyroid Hormones on Kidney Function in Patients After
www.nature.com/scientificreports OPEN Efect of Thyroid Hormones on Kidney Function in Patients after Kidney Transplantation Benjamin Schairer1, Viktoria Jungreithmayr2, Mario Schuster2, Thomas Reiter 1, Harald Herkner3, Alois Gessl4, Gürkan Sengölge1* & Wolfgang Winnicki 1 Elevated levels of thyroid-stimulating-hormone (TSH) are associated with reduced glomerular fltration rate (GFR) and increased risk of developing chronic kidney disease even in euthyroid patients. Thyroid hormone replacement therapy has been shown to delay progression to end-stage renal disease in sub-clinically hypothyroid patients with renal insufciency. However, such associations after kidney transplantation were never investigated. In this study the association of thyroid hormones and estimated GFR (eGFR) in euthyroid patients after kidney transplantation was analyzed. In total 398 kidney transplant recipients were assessed retrospectively and association between thyroid and kidney function parameters at and between defned time points, 12 and 24 months after transplantation, was studied. A signifcant inverse association was shown for TSH changes and eGFR over time between months 12 and 24 post transplantation. For each increase of TSH by 1 µIU/mL, eGFR decreased by 1.34 mL/min [95% CI, −2.51 to −0.16; p = 0.03], corresponding to 2.2% eGFR decline, within 12 months. At selected time points 12 and 24 months post transplantation, however, TSH was not associated with eGFR. In conclusion, an increase in TSH between 12 and 24 months after kidney transplantation leads to a signifcant decrease in eGFR, which strengthens the concept of a kidney- thyroid-axis. Interactions between thyroid hormones and kidney function have been suggested in previous studies. -

Understanding Your Blood Test Lab Results
Understanding Your Blood Test Lab Results A comprehensive "Health Panel" has been designed specifically to screen for general abnormalities in the blood. This panel includes: General Chemistry Screen or (SMAC), Complete Blood Count or (CBC), and Lipid examination. A 12 hour fast from all food and drink (water is allowed) is required to facilitate accurate results for some of the tests in this panel. Below, is a breakdown of all the components and a brief explanation of each test. Abnormal results do not necessarily indicate the presence of disease. However, it is very important that these results are interpreted by your doctor so that he/she can accurately interpret the findings in conjunction with your medical history and order any follow-up testing if needed. The Bernards Township Health Department and the testing laboratory cannot interpret these results for you. You must speak to your doctor! 262 South Finley Avenue Basking Ridge, NJ 07920 www.bernardshealth.org Phone: 908-204-2520 Fax: 908-204-3075 1 Chemistry Screen Components Albumin: A major protein of the blood, albumin plays an important role in maintaining the osmotic pressure spleen or water in the blood vessels. It is made in the liver and is an indicator of liver disease and nutritional status. A/G Ratio: A calculated ratio of the levels of Albumin and Globulin, 2 serum proteins. Low A/G ratios can be associated with certain liver diseases, kidney disease, myeloma and other disorders. ALT: Also know as SGPT, ALT is an enzyme produced by the liver and is useful in detecting liver disorders. -

Wellness Labs Explanation of Results
WELLNESS LABS EXPLANATION OF RESULTS BASIC METABOLIC PANEL BUN – Blood Urea Nitrogen (BUN) is a waste product of protein breakdown and is produced when excess protein in your body is broken down and used for energy. BUN levels greater than 50 mg/dL generally means that the kidneys are not functioning normally. Abnormally low BUN levels can be seen with malnutrition and liver failure. Creatinine – a waste product of normal muscle activity. High creatinine levels are most commonly seen in kidney failure and can also been seen with hyperthyroidism, conditions of overgrowth of the body, rhabdomyolysis, and early muscular dystrophy. Low creatinine levels can indicate low muscle mass associated with malnutrition and late-stage muscular dystrophy. Glucose – a simple sugar that serves as the main source of energy in the body. High glucose levels (hyperglycemia) is usually associated with prediabetes and can also occur with severe stress on the body such as surgery or events like stroke or trauma. High levels can also be seen with overactive thyroid, pancreatitis, or pancreatic cancer. Low glucose levels can occur with underactive thyroid and rare insulin- secreting tumors. Electrolytes – Sodium, Calcium, Potassium, Chloride, and Carbon Dioxide are all included in this category. Sodium – high levels of sodium can be seen with dehydration, excessive thirst, and urination due to abnormally low levels of antidiuretic hormone (diabetes insipidus) as well as with excessive levels of cortisol in the body (Cushing syndrome). Low levels of sodium can be seen with congestive heart failure, cirrhosis of the liver, kidney failure, and the syndrome of inappropriate antidiuretic hormone (SIADH). -

Renal Function in Hypothyroidism A
Eishth Arab Conferenceon the Peacefulljses of Atomit Enersv Anman.3-7 December2006 Renal Function in Hypothyroidism A. ShukrallaKhalidr, M. I. Ahmedr,H. M. Elfakir,N. Hassanr,S. M. Suliman2 l. SudanAtomic Energy Commission 2. Universityof Khartoum,Faculty of Medicine 4:i'eJJlJJ"a!cfy i,rlsll .ijl!-l ,-L .1,j...' i.te.: ,tgsilt J,ora 6Jt-iA ,ls".i ,t-Yt .tor-o ,lJti ,irtr* u.i 261,.r,1-.r"-. -a;trjr,:t erl-ntt /.iJIl i3t!l fu+^ .1 -c,,Lll esl>lt L.b 4+ls.2 Abstract Background Hypothyroidisminduces significant changes in the functionof organsystems such as the heart,muscles and brain.Renal function is also influencedby thyroid status.Physiological effects include changes in water andelectrolyte rnetabolism, notably hyponatremia, and reliable alterations of renalhemodynamics, including decrements in renalblood flow, renalplasma flow, glomerularfiltration rate (GFR). Objective Renal function is profoundlyinfluenced by thyroid status;the purposeof the presentstudy was to determinethe relationshipbetween renal functionand thyroid status of patientswith hypothyroidism. Designand Patients In 5 patientswith primaryhypothyroidisrn and control group renal functions are lneasuredby serurncreatinine and glomerular filtrationrate (GFR) usingmodified in diet renaldisease (MDRD) fonnula. Result In hypothyroidism,mean serum creatinineincreased aud mean estimatedGFR decreased,compared to the control group mean serum creatininedecreased and meanestimated GFR hicreased.Tlre hy,potliyroid patientsshowed elevated serum creatinine levels (> 1.1mg/dl)compared -

Factors Associated with Metabolic Acidosis in Patients Receiving Parenteral Nutrition
NEPHROLOGY 2007; 12, 3–7 doi:10.1111/j.1440-1797.2006.00748.x Original Article Factors associated with metabolic acidosis in patients receiving parenteral nutrition I-CHIEH TSAI,1 JENQ-WEN HUANG,2 TZONG-SHINN CHU,2 KWAN-DUN WU2 and TUN-JUN TSAI2 1Department of Internal Medicine, Taipei Hospital, Department of Health, Hsin-Chuang City and 2National Taiwan University Hospital, Taipei, Taiwan SUMMARY: Aims: Metabolic acidosis is a common problem after infusion with chloride-based parenteral nutrition. However, it is unknown whether the occurrence of metabolic acidosis is related to this regimen of therapy or to patient- specific risk factors. Methods: Patients receiving parenteral nutrition from July to December 2003 at this hospital were included for a retrospective study. Patients were excluded who had illnesses that were potentially related to acid-base disorders. The remaining patients were divided on the basis of parental nutrition they had received: a chloride- base regimen group, and an acetate-based therapy group. Biochemical character and blood gas data were analysed. Continuous variables were analysed by t-test. Categorical variables were assessed by chi-squared test. Independent determinants for bicarbonate decline were analysed using forward stepwise multiple linear regression analysis. Results: There were 29 patients (17 women, 12 men) who received chloride-based regimen and 26 patients (16 women, 10 men) took acetate-based therapy. The acetate group had significantly higher baseline serum creatinine and blood urea nitrogen than chloride group. The blood pH, CO2, bicarbonate and base excess were significantly lower after receiving chloride-based therapy; while these changes were not observed in acetate- based therapy group. -

Evaluating Relationships Atnong Blood Glucose, Plastna Urea Nitrogen, Perfortnance, Morbidity, and Mortality in High-Risk Feedlot Heifers
PEER REVIEWED Evaluating Relationships atnong Blood Glucose, Plastna Urea Nitrogen, Perfortnance, Morbidity, and Mortality in High-risk Feedlot Heifers 3 H.J. Donley1, MS; C. D. Reinhardt2, PhD; J. J. Sindt , PhD; S. P. Montgomery4, PhD; J. S. Drouillard2*, PhD 1Beef Marketing Group, Great Bend, KS 67530 2Department ofAnimal Sciences and Industry, Kansas State University, Manhattan, KS 66506 3VetLife, West Des Moines, IA 50265 4Dekalb Feeds, Rock Falls, IL 61071 *Correspondence: Dr. Drouillard, tel: 785-532-1204; fax 785-532-5681; [email protected] Abstract Resume Two experiments were conducted using crossbred Deux experiences ont ete menees avec des taures beef heifers (Exp. 1: n = 332, 403 lb [183 kg] BW; Exp. de boucherie de race melangee (Exp. 1: n=332, 403 lb 2: n = 334, 456 lb [207 kg] BW) to evaluate relationships [183 kg] masse corporelle; Exp. 2: n=334, 456 lb [207 between blood glucose, plasma urea nitrogen, average kg] masse corporelle) afin d'evaluer la relation des taux daily gain (ADG), and morbidity and mortality rates. de mortalite et de morbidite en fonction du glucose san Whole blood glucose (Glc) was measured 24 hours after guin, de l'azote ureique plasmatique et du gain moyen arrival using a handheld Glc monitor. Plasma was quotidien. La concentration du glucose sanguin etait obtained from 179 heifers in Exp. 1 and from 334 heif mesuree 24 heures apres !'arrive avec un moniteur de ers in Exp. 2 to determine concentrations of plasma glucose portatif. L'analyse spectrometrique du plasma urea nitrogen (PUN) and plasma Glc measured using de 179 taures dans !'experience 1 et de 334 taures dans a spectrophotometer. -
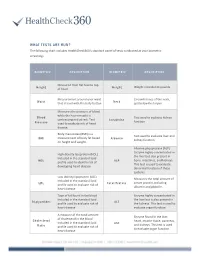
WHAT TESTS ARE RUN? the Following Chart Includes Healthcheck360’S Standard Panel of Tests Conducted at Your Biometric Screening
WHAT TESTS ARE RUN? The following chart includes HealthCheck360’s standard panel of tests conducted at your biometric screening: BIOMETRIC DESCRIPTION BIOMETRIC DESCRIPTION Measured from flat foot to top Height Weight Weight recorded in pounds of head Measurement around your waist Circumference of the neck, Waist Neck that is level with the belly button just below the larynx Measures the pressure of blood while the heart muscle is Blood Test used to evaluate kidney contracting and at rest. Test Creatinine function Pressure used to evaluate risk of heart disease. Body mass index (BMI) is a Test used to evaluate liver and BMI measurement of body fat based Albumin kidney function on height and weight. Alkaline phosphatase (ALP) Enzyme highly concentrated in High-density lipoprotein (HDL) the liver but also present in Included in the standard lipid HDL ALP bone, intestines, and kidneys. profile used to identify risk of This test is used to evaluate developing heart disease abnormal function of these systems. Low-density lipoprotein (LDL) Measures the total amount of Included in the standard lipid LDL Total Protein serum protein, including profile used to evaluate risk of albumin and globulin. heart disease Type of fat found in the blood Enzyme highly concentrated in Included in the standard lipid the liver but is also present in Triglycerides ALT profile used to evaluate risk of the kidneys. This test is used to heart disease evaluate organ function. A measure of the total amount Enzyme found in the liver, of cholesterol in the blood Cholesterol heart, muscle tissue, pancreas, Included in the standard lipid AST and kidneys. -

Hypoalbuminemia Predicts Intensive Care Need Among Adult Inpatients with Community Acquired Pneumonia: a Cross Sectional Study
Original Article Hypoalbuminemia predicts intensive care need among adult inpatients with community acquired pneumonia: a cross sectional study Muhammad Adnan1, Naheed Hashmat2, Muhammad Latif3, Zahra Ali1, Tayyaba Rahat1 1 PHRC Research Center, Fatima Jinnah Medical University, Lahore, Pakistan 2 Departments of Medicine, Sir Ganga Ram Hospital, Lahore, Pakistan 3 Departments of Medicine, Akhtar Saeed Medical and Dental College, Lahore, Pakistan Abstract Introduction: Various scales helped physicians to decide the site of care of pneumonia patients, but had certain limitations. Literature review suggested that serum albumin and B/A ratio predict the site of care but more evidences were required. Therefore, the study was aimed to evaluate the role of serum albumin and B/A ratio in the prediction of intensive care need among patients with community acquired pneumonia. Methodology: The cross-sectional analytical study enrolled 134 adult inpatients with pneumonia from Sir Ganga Ram Hospital, Lahore, Pakistan during September 2014 to December 2016. Serum albumin, creatinine and urea levels were estimated; and BUN, B/A ratio and CURB- 65 scores were calculated to predict the need of ICU. Results: Overall mean age was 50 ± 21 years, and 54.5% patients were females. The patients (19.4%) who required treatment in ICU had significantly lower albumin levels (p = 0.001); elevated BUN levels (p = 0.003), B/A ratio (p = 0.001) and CURB-65 score (p = 0.038); and longer hospital stay (p = 0.002). Hypoalbuminemia showed significant association with the requirement of ICU (OR: 7.956; p = 0.001). The optimal cut-off point of serum albumin to predict ICU need was 3.4 g/dL (50% sensitivity; 89% specificity). -

The Role of Albumin Level and Blood Urea Nitrogen
y & R ar esp on ir m a l to u r y AKPINAR et al., J Pulm Respir Med 2013, 3:5 P f M o e l Journal of Pulmonary & Respiratory d a i DOI: 10.4172/2161-105X.1000159 n c r i n u e o J ISSN: 2161-105X Medicine Research Article Open Access The Role of Albumin Level and Blood Urea Nitrogen/ Albumin Ratio in Prediction of Prognosis of Community Acquired Pneuomonia Evrim Eylem AKPINAR MD¹, Derya HOŞGÜN MD¹, Beyza DOĞANAY PhD², Meral GÜLHAN Prof¹ ¹Ufuk University, Department of Chest Diseases, Ankara, Turkey ²Ankara University, Department of Biostatistics, Ankara, Turkey Abstract Background and aim: Community-Acquired Pneumonia (CAP) is an important cause of morbidity and mortality worldwide. The accuracy of scoring scales is lower regarding the evaluation of mortality and need for ICU treatment. This study aimed to evaluate the role of the albumin level and Blood urea nitrogen /Albumin (BUN/Alb) ratio in the prediction of disease severity and one-month mortality. Materials and methods: Patients hospitalized for CAP were included. Venous blood samples were obtained to measure albumin levels and calculate the ratio of BUN/Alb. The correlations of serum albumin levels and BUN/Alb ratio with the requirement of ICU, development of complications and one-month mortality were evaluated. Results: 216 patients were enrolled. Patients who needed ICU treatment had a higher BUN/Alb ratio (p=0.029). The cut-off level of BUN/Alb in prediction of ICU need was found 4.15 (sensitivity 76%, specificity 49%). Low albumin level was an independent risk factor for ICU need (OR: 5.263, 95% CI: 1.996 to 13.889, p=0.001). -
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel CHOLESTEROL and TRIGLYCERIDES are fats necessary for normal cell function. However, elevated levels of these fats have been associated with an increased risk of developing coronary disease, arteriosclerosis, and heart attack. A patient’s dietary status, medications, presence of illness, lifestyle, and family history may represent factors influencing cholesterol levels. The signifi- cance of cholesterol levels should be determined within the context of each individual patient. If your cholesterol level is 200 mg/dl or greater, please consult your physician. Triglyceride levels greater than 150 mg/dl, in a true fasting specimen, are considered elevated. In this case, please consult your physician. HDL (High-Density Lipoprotein) CHOLESTEROL, commonly known as the “GOOD” cholesterol, picks up cholesterol and transports it for removal from the body. The higher the HDL value, the lower the risk of developing coronary disease, arterioscle- rosis, and heart attack. LDL (Low-Density Lipoprotein) CHOLESTEROL, commonly known as the “BAD” cholesterol, picks up cholesterol and transports it to the cells of the body for storage. Desirable LDL levels are less than 130 mg/dl. The higher the LDL value, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. CHOLESTEROL/HDL RATIO is a calculation used to predict an increased or decreased risk of cardiovascular disease relative to a normal. The higher the ratio, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. GLUCOSE, commonly called a blood sugar, is the transport form of carbohydrates in the body as they move to storage or to utiliza- tion. -
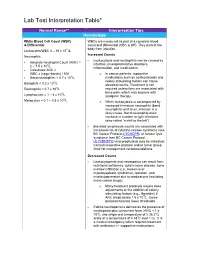
Lab Test Interpretation Table*
Lab Test Interpretation Table* Normal Range** Interpretation Tips Hematology White Blood Cell Count (WBC) WBCs are measured as part of a complete blood & Differential count and differential (CBC & diff). They protect the body from infection. Leukocytes/WBC 4 – 10 x 109 /L Increased Counts Neutrophils - Leukocytosis and neutrophilia can be caused by Absolute Neutrophil Count (ANC) = - infection, myeloproliferative disorders, 2 – 7.5 x 109/L inflammation, and medications. - Calculated ANC = WBC x (segs+bands) / 100 o In cancer patients, supportive - Band neutrophils: < 0.7 x 109/L medications such as corticosteroids and 9 colony stimulating factors can cause Basophils < 0.2 x 10 /L elevated counts. Treatment is not Eosinophils < 0.7 x 109/L required unless they are associated with 9 bone pain, which may improve with Lymphocytes = 1 – 4 x 10 /L analgesic therapy. 9 Monocytes = 0.1 – 0.8 x 10 /L o When leukocytosis is accompanied by increased immature neutrophils (band neutrophils) and fever, infection is a likely cause. Band neutrophils often increase in number to fight infections (also called “a shift to the left”). - Elevated lymphocyte counts are associated with increased risk of cytokine-release syndrome (see BC Cancer Protocol LYCHOPR) or tumour lysis syndrome (see BC Cancer Protocol ULYVENETO) and prophylaxis may be indicated. Consult respective protocol and/or tumor group chair for management recommendations. Decreased Counts - Leukocytopenia and neutropenia can result from nutritional deficiency, autoimmune disease, bone marrow infiltration (i.e., leukemia or myelodysplastic syndrome), radiation, and myelosuppression due to medications (including many cancer drugs). o Many treatment protocols require dose adjustments or the addition of colony stimulating factors (e.g., filgrastim) if ANC drops below 1.5 x 109/L. -

Blood Result Explanation
BLOOD RESULT EXPLANATION SCREENING RESULTS CHLORIDE is also one of the body’s minerals, involved with water balance, most body chloride comes from salt in the diet. A high chlo- Screening results that fall OUTSIDE of the reference range will have ride level may mean severe dehydration, certain kidney disorders a H (High) or L(Low) in the flag column. The Reference range for or hyperventilation. A low chloride level may result from excessive each test is listed on the right side of your blood chemistry report. vomiting, diarrhea, severe burns, excessive sweating or kidney fail- ure. Borderline low or high levels of chloride have very little signifi- Screening values that are OUTSIDE of the Reference ranges: cance. 1. May show you had eaten shortly before blood was drawn. 2. May mean there was a problem with drawing your blood. 3. May indicate possible problems needing medical evaluation. DIABETES SCREEN GLUCOSE is the primary energy source for all body tissues. IT IS NOT POSSIBLE TO DIAGNOSE OR TREAT ANY DISEASE The sugars and carbohydrates you eat are ordinarily converted into OR HEALTH PROBLEM WITH THIS BLOOD SCREEN ALONE. glucose, which can be either used to produce immediate energy, It can help you learn more about your body and detect potential stored in the liver or as fat throughout the body. High blood glucose problems in early stages when treatment or changes in personal (hyperglycemia) after fasting for 12 hours suggests diabetes. Your health habits can be most effective. doctor may want to do further testing. A low glucose level (hypo- glycemia) accompanied with symptoms such as weakness, nausea, sweating and difficulty thinking clearly, is suggestive for hypoglyce- BLOOD RESULTS mia.