Download Preprint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Urgent Statement from Hospitals in the City of Toronto and the Regions of Durham, Halton, Peel and York GTA Hospitals Support Further Lockdown Measures
An Urgent Statement from Hospitals in the City of Toronto and the Regions of Durham, Halton, Peel and York GTA Hospitals Support Further Lockdown Measures December 20, 2020 - Today, on behalf of our patients, health care workers and staff, we are supporting the Ontario Hospital Association’s call for stronger lockdown measures from the Government of Ontario. Across the Greater Toronto Area, COVID-19 infections are continuing to rise, as are hospitalizations and intensive care cases. These trends show no sign of slowing – in fact, a surge in cases following the holiday season is expected to make the situation even worse. Our staff are caring for increasing numbers of COVID-19 patients in hospitals and assisting in other settings such as long-term care homes. They are also keeping up with the care needs of patients without COVID-19 and trying to make progress in the significant backlog of scheduled surgeries and procedures cancelled during the first wave. For many months now, these frontline health care workers have been devoting enormous energy and skill to caring for their patients, at the very epicenter of the pandemic. They are stressed and overstretched. This level of strain is simply not sustainable for much longer. We are seeing increasing numbers of staff becoming ill and not able to work – both with COVID-19 and other illnesses. While we are coping and planning for redeployment, we are seeing more illness and stress and hearing about the toll this is taking on people’s families. We recognize that lockdown measures are challenging for many members of our communities, but we cannot afford to put patients and health care workers at further risk. -

MB-01 COVER.Indd
SHANAH TOVAH uc,f, vcuy vbak INFLUENCERS Plus: Fiction by Ella Burakowski M THE CANADIAN JEWISH NEWS B2 [ RH 5776 ] SEPTEMBER 10, 2015 Supreme Court judge broke new ground A colourful life Employment, she coined the term and in the spotlight the concept of “employment equity,” as a strategy to remedy workplace dis- arbara Amiel has been called a lot of crimination faced by women, Aborigin- B things, but boring shouldn’t be one of al Peoples, people with disabilities and them. visible minorities. Known for her outspoken, politically That same year she was the first conservative column in Maclean’s maga- woman chair of the Ontario Labour Re- zine as much as for her marriage to for- lations Board and later became the first mer media baron Conrad Black, Amiel is Barbara Amiel Rosalie Silberman Abella woman in the British Commonwealth to a British Canadian journalist, writer and head a law reform commission. socialite. In 2001, Amiel made a splash when she osalie Silberman Abella, the first In 2004, she was appointed to the Su- Born in England, Amiel moved with her reported in the British weekly magazine, R Jewish woman appointed to the Su- preme Court, where she has written de- family to Hamilton, Ont., as an adolescent, The Spectator, that the then-French am- preme Court of Canada has been shat- cisions on family law, employment law, but spent years living on her own and bassador to Britain had called Israel “that tering the glass ceiling her entire life. youth criminal justice and human rights. holding various jobs to support herself af- shitty little country” to Black at a private Born to Holocaust survivor parents in She continues to be involved in issues ter her mother and stepfather pushed her dinner party he was hosting. -
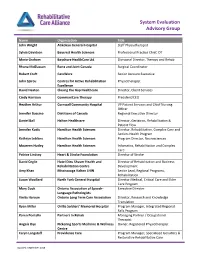
System Evaluation Advisory Group
System Evaluation Advisory Group Name Organization Title John Wright Atikokan General Hospital Staff Physiotherapist Sylvia Davidson Baycrest Health Sciences Professional Practice Chief, OT Marie Graham Bayshore HealthCare Ltd. Divisional Director, Therapy and Rehab Rhona McGlasson Bone and Joint Canada Surgical Coordinator Robert Craft CareWorx Senior Account Executive John Spirou Centres for Active Rehabilitation Physiotherapist Excellence David Heaton Closing the Gap Healthcare Director, Client Services Cindy Harrison CommuniCare Therapy President/CEO Heather Arthur Cornwall Community Hospital VP Patient Services and Chief Nursing Officer Jennifer Buccino Dietitians of Canada Regional Executive Director Daniel Ball Halton Healthcare Director, Geriatrics, Rehabilitation & Patient Flow Jennifer Kodis Hamilton Health Sciences Director, Rehabilitation, Complex Care and Seniors Health Program Kathryn Leblanc Hamilton Health Sciences Program Director, Neurosciences Maureen Hutley Hamilton Health Sciences Infomatics, Rehabilitation and Complex Care Patrice Lindsay Heart & Stroke Foundation Director of Stroke David Ceglie Hotel Dieu Shaver Health and Director of Rehabilitation and Business Rehabilitation Centre Development Amy Khan Mississauga Halton LHIN Senior Lead, Regional Programs, Rehabilitation Susan Woollard North York General Hospital Director Medical, Critical Care and Elder Care Program Mary Cook Ontario Association of Speech- Executive Director Language Pathologists Vinita Haroun Ontario Long Term Care Association Director, Research -

E-Health 2019 Program
Celebrate, Grow & Inspire BOLD ACTION IN CANADA’S DIGITAL HEALTH COMMUNITY May 26 - 29, 2019 Toronto, ON Beanfield Centre Conference Program www.e-HealthConference.com #eHealth2019 eHealthconference @eHealthConf Join Canada’s Largest National e-Health Conference & Tradeshow e-Health 2019 MOBILE APP Download the Whova – Event & Conference App from the App Store or Google Play Store and search ‘e-Health’ to download the e-Health 2019 mobile app Get 24-hour access to all Conference related information including abstract summaries Share your e-Health experience online Earn a spot on the Leaderboard for a chance to win a prize Schedule 'Connecting Minds' Meetings (Discussion Huddles & One-on-One Meetings) Download for Free! www.e-HealthConference.com #eHealth2019 eHealthconference @eHealthConf Table of Contents Welcome to e-Health 2019 ................................ 4 Meet the Plenary Speakers.........................15-16 Conference Hosts ............................................... 5 Conference Program ....................................... 17- General Information........................................... 6 Sunday, May 26, 2019................................. 18 Monday, May 27, 2019 ..........................19-26 Accreditation ....................................................... 7 Tuesday, May 28, 2019 ..........................27-35 Conference Sponsors ...................................... 8-9 Wednesday, May 29, 2019 ....................36-38 What’s New at e-Health 2019 .......................... 10 Author Index.................................................39-45 -

Hospice Association of Ontario
2017 Annual Conference April 23 - 25, 2017 POSTERS P1: JIRA: A NOVEL APPROACH TO A COMMUNITY PALLIATIVE CARE ELECTRONIC MEDICAL RECORD Emilie Lam, MD CCFP, Markham Stouffville Hospital, Markham, ON; Andrew Patterson, MD CCFP(PC), Markham Stouffville Hospital, Markham, ON P2: DEATH AT HOME: FROM DIAGNOSIS OF LIFE-LIMITING ILLNESS TO CAREGIVER BEREAVEMENT Trish Lafantaisie, RN, BScN, CHPCN(C), Maison Vale Hospice, Sudbury, ON; Barbara Ballantyne, RN, MScN, CONc, CHPCNc, Health Sciences North, Sudbury, ON; Claire Warren, RN, MScN, NP, CHPCN(C), North East Community Care Access Centre, Sudbury, ON P3: RURAL PALLIATIVE CARE COMMUNITY TEAM Ryan Alexander, BA, MSc, Community Care City of Kawartha Lakes - Hospice Services, Lindsay, ON; Jodi Dunn, BScN BA RN, Program Director, Continuing Care Program, Rehabilitation Therapies, Health First & Senor Services, Ross Memorial Hospital, Lindsay, ON P4: IMPLANTABLE CARDIVERTER DEFIBRILLATOR (ICD) DEACTIVATION-SUPPORTING A PAINLESS DEATH Karen Harkness, PhD RN CCN(C) CHFN, Cardiac Care Network (Ontario), Toronto, ON; Elizabeth Berry, RN BHA, Cardiac Care Network (Ontario), Toronto, ON; Stewart Smith, MD FRCPC, Western University, London, ON; Heather Ross, MD, MHSc, FRCPC, FCCS, FACC, University Health Network, Toronto, ON P5: BUILDING PRIMARY-LEVEL PALLIATIVE CARE CAPACITY ACROSS CANADA: PALLIUM CANADA’S LEAP COURSES, PRODUCTS AND INFRASTRUCTURE Kathryn Downer, MSc., EdD, Pallium Canada, Ottawa, ON; Jose Pereira, MBChB, CCFP, MSc, College of Family Physicians of Canada, Toronto, ON; Brady Riordan, MEd, -

2018/2019 Annual Report
Responding to SYSTEM CHANGE 2018/19 ANNUAL REPORT As Ontario reorganizes its health care system, health care providers are intensifying their efforts to integrate care, improve transitions and improve how patients experience care across the system. Rehabilitative care can play an important The GTA Rehab Network is ideally positioned role in these efforts — from reducing to take on this work. Our member organizations length of stay to improving patient flow. represent the breadth of rehabilitative care across But achieving those benefits will require a the acute care, rehabilitative and community coordinated approach across the acute care, sectors. We have a strong record of successful rehabilitative and community sectors. collaborative initiatives. And we are already taking action. Together, we are streamlining and standardizing processes to make rehabilitative care more evidence-based, efficient and responsive to the needs of patients. SYSTEM CHANGE IS UNDERWAY. The Network is helping its members to meet the challenge. ENSURING PRICING REFLECTS IDENTIFYING UNMET NEED BEST PRACTICE CARE IN OUTPATIENT REHAB Working with its members, the Network used best practice For years, a lack of data for outpatient rehabilitative care has made system- models of care and outpatient rehab data to propose alternate wide planning difficult. To address this gap, the Network worked with hospital-based outpatient pricing for the Ministry of Health and seven hospitals to gather and analyze data on access to outpatient rehab Long-Term Care hip and knee bundled care pilot. The proposed services for all population groups. The Network is using this data to produce pricing more accurately reflects the resources used by providers a scan of demand and unmet need that will support organizations and the to provide best practice rehabilitative care. -

2–9 November Collaboration
2–9 November Collaboration Presented by Sarah and Chaim Neuberger Holocaust Education Centre UJA Federation of Greater Toronto We Gratefully Acknowledge Our Donors and Sponsors Holocaust Education Week 2014 presenting sponsor lead benefactors The Elizabeth & Tony Comper Foundation Honey & Barry Sherman Holocaust Education Week 2014 explores the distinct ways in which individuals, groups and governments collaborated during the Shoah. media sponsors This inclusive program will address many forms of collaboration: from the experi- ences of those who purposely chose to collaborate with the Nazis in genocide and crimes against humanity—precipitating events such as Kristallnacht and the Hungarian deportations—to those who defied the Nazis and collaborated instead corporate benefactors in resistance and even rescue, as in the Kindertransport, and by those now desig- nated as Righteous Among the Nations. Collaboration serves as a prism for exam- ining the breadth and depth of human and institutional responses to the rise of National Socialism and the events of the Holocaust. foundation, cultural & civic benefactors HEW 2014 is proud to present a group of outstanding experts-in-residence. Our scholar is Professor Doris Bergen of the University of Toronto, whose essay in this Ministry of Foreign Affairs program guide provides an overview of the theme; educator is Martin Hagmayr of Hungary of the Pedagogical Department, Hartheim Castle, where medical professionals and ordinary clerks collaborated to murder the most vulnerable in society through the -

Use of the Federal Research Support Fund at the University of Toronto
USE OF THE FEDERAL RESEARCH SUPPORT FUND AT THE UNIVERSITY OF TORONTO DEFINITION OF THE INDIRECT COSTS OF RESEARCH When people think about the cost of research, for many what comes to mind are things like specialized equipment, salaries of trainees (graduate students, post-doctoral fellows and research assistants), travel costs, and so forth. These are the “direct” costs of research – budgeted expenditures for undertaking a specific research project. Less likely to be associated with the costs of research, however, are things like the building where the researcher works, the electricity used to power the specialized equipment, or the staff that provide support for research contracts/applications/ oversight and compliance. These “indirect” costs are the hidden costs of research; the real dollars incurred by the University, or affiliated institutions, to support research activities. It can be difficult to assign these costs to specific projects since these necessities for conducting research are often shared. The University of Toronto has established a Research Administration Policy that provides a more specific definition of “direct” and “indirect” costs (http://www.governingcouncil.utoronto.ca/Assets/Governing+Council+Digital+Assets/Policies/Policy/resagree.pdf). DESCRIPTION OF THE RESEARCH SUPPORT FUND PROGRAM (RSF) The Research Support Fund program (RSF: http://www.rsf-fsr.gc.ca/home-accueil-eng.aspx) provides partial support to universities and their affiliates for the indirect costs of research associated with eligible Tri-Council (i.e., NSERC, SSHRC, CIHR) and/or Networks of Centers of Excellence (NCEs) awards. This funding helps institutions to meet the financial burden of hidden costs of research; costs such as lighting, heating, and addressing the increasingly complex oversight and compliance requirements that insure the safety of researchers, as well as their human and animal subjects. -

Brain Awareness Week Partner List
Brain Awareness Week Partner List Brain Awareness Week is the global campaign to foster public enthusiasm and support for brain science. Every March, partners host imaginative activities in their communities that share the wonders of the brain and the impact brain science has on our everyday lives. Partners include colleges and universities, hospitals, medical research institutions, K-12 schools, advocacy groups, outreach organizations, professional associations, government agencies, corporations, and more. Since its founding in 1996, Brain Awareness Week has included the participation of more than 8,200 partners in 124 countries. The map above shows the geographical distribution of Brain Awareness Partners worldwide. Click on a country in the list below to be brought to that section in the Partner List. Georgia Kuwait Norway Spain Albania Chile China Germany Latvia Pakistan Sri Lanka Algeria Hong Kong (China) Ghana Lebanon Palestine Sudan Argentina Macau (China) Greece Lesotho Panama Sweden Armenia Colombia Grenada Liberia Paraguay Switzerland Australia Costa Rica Guatemala Libya Peru Taiwan Austria Côte d’Ivoire Guyana Lithuania Philippines Tanzania Bahamas Croatia Haiti Luxembourg Poland Thailand Bahrain Cuba Hungary Madagascar Portugal Trinidad and Tobago Bangladesh Cyprus Iceland Malaysia Qatar Tunisia Belgium Czech Republic India Malta Romania Turkey Belize Democratic Republic Indonesia Mexico Russia Uganda Benin of the Congo Iran Moldova Saudi Arabia Ukraine Bolivia Denmark Iraq Mongolia Senegal United Arab Emirates Bosnia and -

Baycrest Health Sciences
Baycrest Health Sciences ANNUAL REPORT 2013–14 ANNUAL REPORT 2013–14 B MESSAGE FROM THE BAYCREST BOARD CHAIR AND THE PRESIDENT & CEO Meeting the Changing Needs of Seniors In recent years, the population that Baycrest We recognize the importance of meeting these Health Sciences cares for has changed client-centred needs, and we are responding. dramatically. The older adults who rely on us Technological innovations such as MyChart each and every day are living longer and have are giving clients and their caregivers secure more chronic illnesses, including dementia. access to their Baycrest health records online. They also expect more from healthcare and We have begun to take some of our programs, residential care providers. such as falls prevention and fitness classes, out into the community, allowing seniors to age in As our clients’ needs and expectations place longer. And our activities at Baycrest are change, so too must Baycrest. We know older not just recreational – in many cases they are adults want to be more involved in their own designed to truly help seniors age smarter, and healthcare. They expect direct and ready are based on research and innovative clinical access to their health records, and to have practices being developed right here their families actively participate with them in on our campus. their care plans and treatment decisions. They believe services should be available when Baycrest is a global leader in geriatric care, and where they are most convenient to meet research, education and innovation, and we their needs. Those clients residing in long-term remain so by constantly adapting to provide care or assisted living want more access the greatest possible impact for seniors. -

Practicum Training in Clinical Dietetics at Baycrest Health Sciences
Practicum Training in Clinical Dietetics at Baycrest Health Sciences Baycrest provides practicum placements supervised by Registered Dietitians (RDs) for students enrolled in accredited nutrition and dietetic education programs at Canadian universities. About Baycrest Baycrest Health Sciences is a global leader in geriatric healthcare, residential living, research, innovation, and education, with a special focus on brain health and aging. As an academic health sciences centre fully affiliated with the University of Toronto, Baycrest provides an exemplary care experience for aging clients combined with an extensive clinical training program for students and one of the world’s top research institutes in cognitive neuroscience. Baycrest is located at 3560 Bathurst Street in Toronto near Bathurst and Wilson streets. It is easily accessible by public transit. Care. Baycrest serves approximately 1200 seniors per day. It is home to a globally recognized and innovative continuum of healthcare, wellness, and prevention programs and services. Services include outpatient clinics, a hospital, long-term care home, and residential and community-based programs designed especially for people in their 50s, 60s, 70s, 80s and beyond. Research & Innovation. Baycrest is a leader in cognitive neuroscience and memory research, with the goal of transforming the journey of aging. The stellar reputation of the Rotman Research Institute reflects its ability to raise and answer fundamental questions about memory, aging, and the neuroscience of cognition. The Kunin-Lunenfeld Applied & Evaluative Research Unit provides resources and expertise to support clinical, evaluative, and translational research at Baycrest. The Canadian Centre for Aging and Brain Health Innovation is a solution accelerator focused on driving innovation in the aging and brain health sector. -

The DWQ-EMR Embedded Tool to Enhance the Family Physician-Caregiver Connection: a Pilot Case Study
geriatrics Article The DWQ-EMR Embedded Tool to Enhance the Family Physician-Caregiver Connection: A Pilot Case Study Kristina Marie Kokorelias 1 , Einat Danieli 2, Sheila Dunn 3, Sid Feldman 2, David Patrick Ryan 4 and Joel Sadavoy 5,* 1 St. John’s Rehab Research Program, Sunnybrook Health Sciences Research Institute, Sunnybrook Health Sciences Centre, 285 Cummer Avenue, Room B105, Toronto, ON M2M 2G1, Canada; [email protected] 2 Baycrest Health Sciences, 3560 Bathurst St, Toronto, ON M6A 2E1, Canada; [email protected] (E.D.); [email protected] (S.F.) 3 Women’s College Research Institute, Women’s College Hospital, 790 Bay St, Toronto, ON M5G 1N8, Canada; [email protected] 4 Faculty of Medicine, University of Toronto, 263 McCaul St 3 Fl, Toronto, ON M5T 1W7, Canada; [email protected] 5 Department of Psychiatry, Sinai Health, 60 Murray St, Toronto, ON M5P 3C3, Canada * Correspondence: [email protected]; Tel.: +1-416-586-4800 Abstract: The number of family caregivers to individuals with dementia is increasing. Family physicians are often the first point of access to the health care system for individuals with dementia and their caregivers. Caregivers are at an increased risk of developing negative physical, cognitive and affective health problems themselves. Caregivers also describe having unmet needs to help them sustain care in the community. Family physicians are in a unique position to help support caregivers and individuals with dementia, but often struggle with keeping up with best practice dementia service knowledge. The Dementia Wellness Questionnaire was designed to serve as a starting point Citation: Kokorelias, K.M.; Danieli, for discussions between caregivers and family physicians by empowering caregivers to communicate E.; Dunn, S.; Feldman, S.; Ryan, D.P.; their needs and concerns and to enhance family physicians’ access to specific dementia support Sadavoy, J.