Download Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Molecular Phylogenetics and Evolution 123 (2018) 59–72
Molecular Phylogenetics and Evolution 123 (2018) 59–72 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenetic relationships and cryptic species diversity in the Brazilian egg- T brooding tree frog, genus Fritziana Mello-Leitão 1937 (Anura: Hemiphractidae) ⁎ Marina Walker1, , Mariana L. Lyra1, Célio F.B. Haddad Universidade Estadual Paulista, Instituto de Biociências, Departamento de Zoologia and Centro de Aquicultura (CAUNESP), Campus Rio Claro, Av. 24A,No 1515, Bela Vista, CEP 13506-900 Rio Claro, São Paulo, Brazil ARTICLE INFO ABSTRACT Keywords: The genus Fritziana (Anura: Hemiphractidae) comprises six described species (F. goeldii, F. ohausi, F. fissilis, F. Egg-brooding frogs ulei, F. tonimi, and F. izecksohni) that are endemic to the Brazilian Atlantic Forest. Although the genus has been Molecular phylogeny the subject of studies dealing with its taxonomy, phylogeny, and systematics, there is considerable evidence for Brazilian Atlantic Forest cryptic diversity hidden among the species. The present study aims to understand the genetic diversity and Species diversity phylogenetic relationships among the species of Fritziana, as well as the relationships among populations within New candidate species species. We analyzed 107 individuals throughout the distribution of the genus using three mitochondrial gene Mitochondrial gene rearrangements fragments (12S, 16S, and COI) and two nuclear genes (RAG1 and SLC8A3). Our data indicated that the species diversity in the genus Fritziana is underestimated by the existence of at least three candidate species hidden amongst the group of species with a closed dorsal pouch (i.e. F. fissilis and F. ulei). We also found four species presenting geographical population structures and high genetic diversity, and thus require further investigations. -

Anura: Hemiphractidae: Gastrotheca)
Accepted Manuscript Short communication Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca) David C. Blackburn, William E. Duellman PII: S1055-7903(13)00179-6 DOI: http://dx.doi.org/10.1016/j.ympev.2013.04.021 Reference: YMPEV 4580 To appear in: Molecular Phylogenetics and Evolution Received Date: 7 January 2013 Revised Date: 2 April 2013 Accepted Date: 22 April 2013 Please cite this article as: Blackburn, D.C., Duellman, W.E., Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca), Molecular Phylogenetics and Evolution (2013), doi: http://dx.doi.org/10.1016/ j.ympev.2013.04.021 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Short Communication 2 3 Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca) 4 5 David C. Blackburna,*, William E. Duellmanb 6 a Department of Vertebrate Zoology & Anthropology, California Academy of Sciences, 55 7 Music Concourse Drive, San Francisco, CA 94118, USA 8 b Biodiversity Institute, University of Kansas, 1345 Jayhawk Boulevard, Lawrence, KS 9 66045, USA 10 * Corresponding author. E-mail address: [email protected] (D.C. Blackburn) 11 12 Abstract 13 Molecular phylogenetic analyses based on expanded taxonomic and geographic sampling 14 support the monophyly of the marsupial frog genera (family Hemiphractidae), resolve six 15 geographically circumscribed lineages within Gastrotheca, and, for the first time, reveal 16 that two divergent lineages of Gastrotheca inhabit the Atlantic Coastal Forests of Brazil. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Zootaxa, Herpetological Results of the 2002 Expedition to Sarisarinama, A
ZOOTAXA 1942 Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species CESAR L. BARRIO-AMOROS & CHARLES BREWER-CARIAS Magnolia Press Auckland, New Zealand Cesar L. Barrio-Amoros & Charles Brewer-Carias Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species (Zootaxa 1942) 68 pp.; 30 cm. 26 Nov. 2008 ISBN 978-1-86977-269-7 (paperback) ISBN 978-1-86977-270-3 (Online edition) FIRST PUBLISHED IN 2008 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2008 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 1942 © 2008 Magnolia Press BARRIO-AMORÓS & BREWER-CARÍAS Zootaxa 1942: 1–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species CÉSAR L. BARRIO-AMORÓS1 & CHARLES BREWER-CARÍAS2 1Fundación AndígenA, Apartado Postal 210, 5101-A Mérida, Venezuela. -
Compare These Types of Parental Care in Non-‐Amniotes
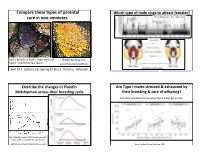
5/4/16 Compare these types of parental Which type of male sings to a6ract females? care in non-‐amniotes. McIver, Marchaterre,. Rice, & Bass, 2014 Time (sec) Type I: 80-‐90% of males, large, territorial Plainfin Midshipman Type II: small body, fast sperm hOp://www.basslab.org/photos/ hOp://bio3520.nicerweb.com/Locked/chap/ch03/sonic_muscles.html Biol 417, Lecture 16, Spring 2016, Dr. Karen E. Petersen hOp://blogs.scienQficamerican.com/brainwaves/what-‐singing-‐fish-‐reveal-‐about-‐speech-‐and-‐hearing/ hOp://www.livescience.com/27237-‐fish-‐sings-‐for-‐mates.html Describe the changes in Plainfin Are Type I males stressed & exhausted by Midshipman across their breeding cycle their breeding & care of offspring? ScienQsts wanted to know when these males get to eat…. (A) CollecQon depth & (B) GonadosomaQc index (GSI) of females & type I males Forlano, Sisneros, Rohmann, & Bass, 2015 Bose, CogliaQ, Howe, Balshine, 2014 1 5/4/16 What proporEon of Type I males Paternal care occurs in Glass Frogs engaged in cannibalism of eggs? Obligate vs. facultaQve care Grey bars: cannibalisQc males; white bars: noncannibalisQc males Hyalinobatrachium fleischmanni, Family Centrolenidae Delia, Ramírez-‐BauQsta, & Summers, 2014 Bose, CogliaQ, Howe, Balshine, 2014 hOp://www.sciencemag.org/news/2014/04/when-‐dads-‐go-‐missing-‐frogs-‐start-‐hatching Biparental care, monogamy & matrophy! All/most? Female frogs in the family Ranitomeya imitator, Family DendrobaQdae, the poison dart frogs HemiphracEdae a6ach eggs to their backs Stefania ayangannae Brown, Morales, Summers, 2010 2 5/4/16 Tadpoles or direct development with a6ached eggs A frog that gives birth to tadpoles! Limnonectes larvaepartus, Family Dicroglossidae (Fanged Frogs) Pipa carvalhoi PipaFernandes pipa, Antoniazzi, Sasso-‐Cerri, et al. -

Check List 8(2): 207-210, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(2): 207-210, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Amphibians and Reptiles from Paramakatoi and Kato, PECIES S Guyana OF ISTS 1* 2 L Ross D. MacCulloch and Robert P. Reynolds 1 Royal Ontario Museum, Department of Natural History. 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada. 2 National Museum of Natural History, USGS Patuxent Wildlife Research Center, MRC 111, P.O. Box 37012, Washington, D.C. 20013-7012, USA. * Corresponding author. E-mail: [email protected] Abstract: We report the herpetofauna of two neighboring upland locations in west-central Guyana. Twenty amphibian and 24 reptile species were collected. Only 40% of amphibians and 12.5% of reptiles were collected in both locations. This is one of the few collections made at upland (750–800 m) locations in the Guiana Shield. Introduction palm stands (Maurita flexuosa) (Hollowell et al. 2003). The Guiana Shield region of northeastern South America Immediately west of Kato is the nearby Chiung River, is one of the world’s areas of greatest biodiversity. The a rocky-bottomed watercourse about 50 m wide with herpetofauna of the region remains poorly documented, numerous small falls and a distinct riparian zone. Many although there have been several general publications small agricultural clearings, in typical rotating “slash and on the subject (Starace 1998; Gorzula and Señaris 1999; burn” fashion, are common around Kato in the areas where Lescure and Marty 2000; Reynolds et al. 2001; Avila- savanna transitions to forest. -

Amazon Alive!
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Download (Pdf, 101
Natural History Notes REFERENCES TRITURUS CRISTATUS (Great crested newt): PREDATION BY BIRDS. I am fortunate enough Duellman, W. E. & Hoogmoed, M. S. (1984). The to have a pond with a large colony of Great crested taxonomy and phylogenetic relationships of the newts on my land and, over a number of years, have hylid frog genus Stefania . Misc. Publ. Mus. Nat. been able to observe the extensive predation that the Hist. Univ. Kansas 75, 1–39. colony suffers from birds (the pond is in the High Frost, D.R., Grant, T., Faivovich, J., Bain, R., Weald of Kent and lies about half a mile from the Haas, A., Haddad, C.F.B., de Sa´, R.O., River Teise). Donnellan, S.C., Raxworthy, C.J., Wilkinson, I have only been able to find very limited M., Channing, A., Campbell, J.A., Blotto, B.L., references to the fact that this predation occurs and have to assume that it has not been adequately Moler, P., Drewes, R.C., Nussbaum, R.A., recorded or documented in the past. My Lynch, J.D., Green, D. & Wheeler, W.C. (2006). observations and identification of the prey are made The amphibian tree of life. Bull. Am. Mus. Nat. easier by the fact that there are no fish in the pond Hist. 297, 1–370. other than a few large grass-eating carp and no other Jungfer, K-H. & Boehme, W. (1991). The newts are resident. It is also clear that adult newts backpack strategy of parental care in frogs, with are not a problem for some birds despite the belief notes on froglet-carrying in Stefania evansi that toxicity affords some protection. -

2008 Board of Governors Report
American Society of Ichthyologists and Herpetologists Board of Governors Meeting Le Centre Sheraton Montréal Hotel Montréal, Quebec, Canada 23 July 2008 Maureen A. Donnelly Secretary Florida International University Biological Sciences 11200 SW 8th St. - OE 167 Miami, FL 33199 [email protected] 305.348.1235 31 May 2008 The ASIH Board of Governor's is scheduled to meet on Wednesday, 23 July 2008 from 1700- 1900 h in Salon A&B in the Le Centre Sheraton, Montréal Hotel. President Mushinsky plans to move blanket acceptance of all reports included in this book. Items that a governor wishes to discuss will be exempted from the motion for blanket acceptance and will be acted upon individually. We will cover the proposed consititutional changes following discussion of reports. Please remember to bring this booklet with you to the meeting. I will bring a few extra copies to Montreal. Please contact me directly (email is best - [email protected]) with any questions you may have. Please notify me if you will not be able to attend the meeting so I can share your regrets with the Governors. I will leave for Montréal on 20 July 2008 so try to contact me before that date if possible. I will arrive late on the afternoon of 22 July 2008. The Annual Business Meeting will be held on Sunday 27 July 2005 from 1800-2000 h in Salon A&C. Please plan to attend the BOG meeting and Annual Business Meeting. I look forward to seeing you in Montréal. Sincerely, Maureen A. Donnelly ASIH Secretary 1 ASIH BOARD OF GOVERNORS 2008 Past Presidents Executive Elected Officers Committee (not on EXEC) Atz, J.W. -

3Systematics and Diversity of Extant Amphibians
Systematics and Diversity of 3 Extant Amphibians he three extant lissamphibian lineages (hereafter amples of classic systematics papers. We present widely referred to by the more common term amphibians) used common names of groups in addition to scientifi c Tare descendants of a common ancestor that lived names, noting also that herpetologists colloquially refer during (or soon after) the Late Carboniferous. Since the to most clades by their scientifi c name (e.g., ranids, am- three lineages diverged, each has evolved unique fea- bystomatids, typhlonectids). tures that defi ne the group; however, salamanders, frogs, A total of 7,303 species of amphibians are recognized and caecelians also share many traits that are evidence and new species—primarily tropical frogs and salaman- of their common ancestry. Two of the most defi nitive of ders—continue to be described. Frogs are far more di- these traits are: verse than salamanders and caecelians combined; more than 6,400 (~88%) of extant amphibian species are frogs, 1. Nearly all amphibians have complex life histories. almost 25% of which have been described in the past Most species undergo metamorphosis from an 15 years. Salamanders comprise more than 660 species, aquatic larva to a terrestrial adult, and even spe- and there are 200 species of caecilians. Amphibian diver- cies that lay terrestrial eggs require moist nest sity is not evenly distributed within families. For example, sites to prevent desiccation. Thus, regardless of more than 65% of extant salamanders are in the family the habitat of the adult, all species of amphibians Plethodontidae, and more than 50% of all frogs are in just are fundamentally tied to water. -

Global Diversity of Amphibians (Amphibia) in Freshwater
Hydrobiologia (2008) 595:569–580 DOI 10.1007/s10750-007-9032-2 FRESHWATER ANIMAL DIVERSITY ASSESSMENT Global diversity of amphibians (Amphibia) in freshwater Miguel Vences Æ Jo¨rn Ko¨hler Ó Springer Science+Business Media B.V. 2007 Abstract This article present a review of species amphibians is very high, with only six out of 348 numbers, biogeographic patterns and evolutionary aquatic genera occurring in more than one of the major trends of amphibians in freshwater. Although most biogeographic divisions used herein. Global declines amphibians live in freshwater in at least their larval threatening amphibians are known to be triggered by phase, many species have evolved different degrees of an emerging infectious fungal disease and possibly by independence from water including direct terrestrial climate change, emphasizing the need of concerted development and viviparity. Of a total of 5,828 conservation efforts, and of more research, focused on amphibian species considered here, 4,117 are aquatic both their terrestrial and aquatic stages. in that they live in the water during at least one life- history stage, and a further 177 species are water- Keywords Amphibia Á Anura Á Urodela Á dependent. These numbers are tentative and provide a Gymnophiona Á Species diversity Á Evolutionary conservative estimate, because (1) the biology of many trends Á Aquatic species Á Biogeography Á Threats species is unknown, (2) more direct-developing spe- cies e.g. in the Brachycephalidae, probably depend directly on moisture near water bodies and (3) the Introduction accelerating rate of species discoveries and descrip- tions in amphibians indicates the existence of many Amphibians are a textbook example of organisms more, yet undescribed species, most of which are living at the interface between terrestrial and aquatic likely to have aquatic larvae.