Modifications of Hemoglobin and Myoglobin by Maillard Reaction Products (Mrps)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Myoglobin Expression Under Hypoxic Condtions In
THESIS IN THE FACE OF HYPOXIA: MYOGLOBIN EXPRESSION UNDER HYPOXIC CONDTIONS IN CULTURED WEDDELL SEAL SKELETAL MUSCLE CELLS Submitted by Michael Anthony De Miranda Jr. Department of Biology In partial fulfillment of the requirements For the degree of Master of Science Colorado State University Fort Collins, Colorado Spring 2012 Master’s Committee: Advisor: Shane Kanatous Gregory Florant Scott Earley Copyright by Michael A. De Miranda Jr. 2012 All Rights Reserved ABSTRACT IN THE FACE OF HYPOXIA: MYOGLOBIN EXPRESSION UNDER HYPOXIC CONDITIONS IN CULTURED WEDDELL SEAL SKELETAL MUSCLE CELLS The hallmark adaptation to breath-hold diving in Weddell seals (Leptonychotes weddellii) is enhanced concentrations of myoglobin in their skeletal muscles. Myoglobin is a cytoplasmic hemoprotein that stores oxygen for use in aerobic metabolism throughout the dive duration. In addition, throughout the duration of the dive, Weddell seals rely on oxygen stored in myoglobin to sustain aerobic metabolism in which lipid is the primary contributor of acetyl CoA for the citric acid cycle. Together, enhanced myoglobin concentrations and a lipid-based aerobic metabolism represent some of the unique adaptations to diving found in skeletal muscle of Weddell seals. This thesis presents data that suggests cultured Weddell seal skeletal muscle cells inherently possess adaptations to diving such as increased myoglobin concentrations, and rely on lipids to fuel aerobic metabolism. I developed the optimum culture media for this unique primary cell line based on myoblast confluence, myoblast growth rates, myotube counts, and myotube widths. Once the culture media was established, I then determined the de novo expression of myoglobin under normoxic and hypoxic oxygen conditions and the metabolic profile of the myotubes under each oxygen condition. -

Genetically Determined Hypoalbuminemia As a Risk Factor for Hypertension: Instrumental Variable Analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2*
www.nature.com/scientificreports OPEN Genetically determined hypoalbuminemia as a risk factor for hypertension: instrumental variable analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2* Hypoalbuminemia is associated with vascular endothelial dysfunction and the development of chronic cardiovascular diseases. However, the relationship between serum albumin concentration and blood pressure changes remains controversial. Community‑based longitudinal cohort data collected from Korean Genome and Epidemiology Study were used in this study. Hypoalbuminemia was defned as a serum albumin concentration of ≤ 4.0 g/dL. A total of 4325 participants were categorized into control (n = 3157) and hypoalbuminemia (n = 1168) groups. Serum albumin had a non‑linear relationship with the risk of hypertension development. A genome‑wide association study revealed 71 susceptibility loci associated with hypoalbuminemia. Among susceptibility loci, genetic variations at rs2894536 in LOC107986598 and rs10972486 in ATP8B5P were related to elevated blood pressure. Serum albumin (HR = 0.654, 95% CI 0.521–0.820) and polymorphisms of rs2894536 (HR = 1.176, 95% CI 1.015–1.361) and rs10972486 (HR = 1.152, 95% CI 1.009–1.316) were signifcant predictors of hypertension development. Increased albumin concentration instrumented by 2 hypoalbuminemia‑associated SNPs (rs2894536 and rs10972486) was associated with decreased HRs for hypertension development (HR = 0.762, 95% CI 0.659–0.882 and HR = 0.759, 95% CI 0.656–0.878). Our study demonstrated that genetically determined hypoalbuminemia is a signifcant predictor of incipient hypertension. Albumin, one of the major serum proteins, has multiple important physiological functions involving stabilization of plasma colloid osmotic pressure, transportation of diverse substances, and signifcant antioxidant activity, and its concentration is fnely regulated by various systems in the physiologic state 1. -

Glycated Hemoglobin and Glycated Albumin in Patients with Diabetes
Kitajima et al. Renal Replacement Therapy (2020) 6:10 https://doi.org/10.1186/s41100-020-0260-5 RESEARCH Open Access Glycated hemoglobin and glycated albumin in patients with diabetes undergoing hemodiafiltration Yukie Kitajima1*, Shunichiro Urabe2, Takashi Hosono2, Satoshi Yoshikawa3, Yuzuru Sato3 and Toru Hyodo2 Abstract Background: Online hemodiafiltration (OHDF), which results in high albumin leakage, is now widely used in Japan for dialysis, since the national insurance system began reimbursing its costs in 2012. Glycated albumin (GA) levels are affected by albumin leakage into effluent dialysate fluid. Therefore, GA levels in patients requiring diabetes- related dialysis undergoing OHDF require monitoring. However, there have been no previous reports on glycemic control indicators of patients with diabetes undergoing OHDF. We aimed to develop a glycemic control index for patients requiring diabetes-related dialysis undergoing OHDF. Methods: This study comprised 133 diabetic patients undergoing OHDF. We examined the correlation between GA and glycated hemoglobin (HbA1c) levels. We analyzed effluent dialysate fluid samples from 41 patients classified into 3 groups, namely, group A, non-protein-leaking OHDF (n = 20); group B, protein-leaking OHDF (n = 14); and group C, highly efficient protein-leaking OHDF (n = 7). We examined the association between GA and HbA1c levels in each group and among patients. Results: A significant positive correlation was observed between GA and HbA1c levels (r = 0.562, p < 0.0001). There was no significant correlation between pre-dialysis blood glucose levels and HbA1c or GA levels as observed on regular blood tests performed under non-fasting conditions. Patients were classified into 2 groups based on their mean albumin levels (3.4 g/dL cutoff). -

Profiling Glycated Hemoglobin Level, Lactate Dehydrogenase And
International Journal of Medical Laboratory 2017;4(2):135-141. Original Article Profiling Glycated Hemoglobin Level, Lactate Dehydrogenase and Alkaline Phosphatase Activity in Gestational Diabetes Mellitus Obese Women and Compare Them with Each Other Mohammadreza Nadimi Barforoushi1M.Sc. , Durdi Qujeq2,3*Ph.D Bostan Roudi1Ph.D. 1Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran. 2Department of Clinical Biochemistry, Faculty of Medicine, Babol University of Medical Sciences, Babol, Iran. 3Cellular and Molecular Biology Research Center (CMBRC), Health Research Institute, Babol University of Medical Sciences, Babol, Iran. A B S T R A C T Article history Background and Aims: The aim of this study was profiling glycated Received 16 Feb 2017 hemoglobin (HbA1c) level, lactate dehydrogenase (LDH) and alkaline Accepted 7 May 2017 phosphatase (ALP) activity in obese women with gestational diabetes Available online 28 Jun 2017 mellitus (GDM) and evaluating the correlation between them. Key words Materials and Methods: Sample size was 90 subjects admitted to the Alkaline phosphatase activity clinical laboratory, who were divided into three groups, in each group Gestational diabetes mellitus (n=30). Subjects glycemic control was checked by HbA1c; ALP, LDH Glycated hemoglobin activity and serum glucose were determined with commercial kit. Age and Lactate dehydrogenase body mass index (BMI) was recorded for each subject. The correlation analysis between blood activity of ALP, LDH activity, HbA1c, glucose, BMI and age in diabetic and normal pregnant women was carried out. Results: The mean of HbA1c level was significantly higher in the GDM obese women than in women with normal pregnancy (p=0.01). In contrast, the means of ALP and LDH activity were lower in the GDM obese women Downloaded from ijml.ssu.ac.ir at 3:56 IRST on Thursday September 30th 2021 than in women with normal pregnancy (p=0.09, and p=0.15, respectively). -

To Study the Co-Relationship Between Glycosylated Hemoglobin and Serum Calcium Levels in Type 2 Diabetes Mellitus Patients
International Journal of Medical and Health Research International Journal of Medical and Health Research ISSN: 2454-9142 Received: 13-01-2020; Accepted: 14-02-2020; Published: 09-03-2020 www.medicalsciencejournal.com Volume 6; Issue 03; 2020; Page No. 43-45 To study the co-relationship between glycosylated hemoglobin and serum calcium levels in type 2 diabetes mellitus patients Dr. Hardeep Singh Deep1, Dr. Jasmine Kaur2, Dr. Gurjyot Singh Nanda3, Dr. Seerat Kaur4 1 Professor MD Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 2 Assistant. Professor MD Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 3 junior resident Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 4 junior resident Radiodiagnosis Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India Abstract Background: The incidence of type-2 Diabetes Mellitus has increased world-wide making it a major public health problem. Electrolyte and mineral abnormalities are common in patients with type-2 Diabetes Mellitus. Therefore, this study was undertaken to look for the correlation between HbA1c (glycated hemoglobin) and serum calcium levels in patients with type-2 Diabetes Mellitus. Aim: To study the co-relationship between glycosylated hemoglobin and serum calcium levels in type 2 diabetes mellitus patients. Materials and Methods: A total of 50 type 2 Diabetic patients and 50 healthy non-Diabetic individuals were included for the study. Both fasting and post prandial blood samples were collected from the two groups and were used for fasting blood sugar, HbA1c, serum calcium, RFT, LFT, CBC, UACR. -

Correlation Between Glycated Hemoglobin and Venous Blood Sugar in Diabetic Patients Monitored in Abidjan
Vol. 14(4), pp. 135-141, October-December 2020 DOI: 10.5897/AJBR2020.1102 Article Number: CD65C6E65033 ISSN 1996-0778 Copyright © 2020 Author(s) retain the copyright of this article African Journal of Biochemistry Research http://www.academicjournals.org/AJBR Full Length Research Paper Correlation between glycated hemoglobin and venous blood sugar in diabetic patients monitored in Abidjan MONDE Aké Absalome1*, CAMARA-CISSE Massara2, KOFFI Konan Gervais2, DIALLO Issiagha3, AKE AKE Alexandre4, YAPO-AKE Bénédicte4, ECRABEY Yann Christian3, KOUAKOU Francisk3, BENE YAO Roger Espérance4 and TIAHOU Georges5 1Félix HOUPHOUËT-BOIGNY University, Cocody, Abidjan, Côte d’Ivoire. 2Biochemistry Laboratory, Abidjan Medical School, Félix HOUPHOUËT BOIGNY University, Côte D'ivoire. 3Biochemistry Laboratory, University Hospital Center of Treichville, Côte D'ivoire. 4Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Félix HOUPHOUËT-BOIGNY University, Côte D'ivoire. 5Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Alassane OUATTARA University, Bouaké, Côte D'ivoire. Received 23 August, 2020; Accepted 2 October, 2020 The aim of this study was to determine the correlation between glycated hemoglobin and blood sugar levels in diabetic subjects carried out in Abidjan. This cross-sectional study included 100 patients with diabetes monitored, for three months, for whom glycated blood glucose and hemoglobin were performed, this after informed consent of the patients. Pearson and Spearman correlation tests were used, at the 5% threshold. The patients with normal HbA1C and normal blood glucose accounted for 55.34 and 32%, respectively. A sedentary lifestyle and body mass index > 25 kg/m² were associated with a significant increase in the risk of increased blood glucose and HbA1C. -

Myoglobin from Equine Skeletal Muscle
Myoglobin from equine skeletal muscle Catalog Number M0630 Storage Temperature –20 C CAS RN 100684-32-0 Precautions and Disclaimer This product is for R&D use only, not for drug, Product Description household, or other uses. Please consult the Safety Molecular mass:1 17.6 kDa Data Sheet for information regarding hazards and safe Extinction coefficient:2 EmM = 12.92 (555 nm) handling practices. pI:3 7.3 (major component) and 6.8 (minor component) Preparation Instructions Myoglobin from horse skeletal muscle is a single chain This protein is soluble in water (10 mg/ml), yielding a heme protein containing 153 amino acid residues. It clear, red brown solution. posesses no disulfide bridges or free -SH groups. Myoglobin contains 8 variously sized right-handed References helical regions, joined by non-ordered or random coil 1. Darbre, P.D. et al., Comparison of the myoglobin of regions. These 8 helices (A, B, C, D, E, F, G, and H) the zebra (Equus burchelli) with that of the horse are folded back on top of one another, and the heme is (Equus cabalus). Biochim. Biophys. Acta, 393(1), situated between helices E and F. The heme is almost 201-204 (1975). totally buried. Only the edge carrying the two 2. Bowen, W.J., The absorption spectra and extinction hydrophylic propionic acid groups is exposed. The coefficients of myoglobin. J. Biol. Chem., 179, 235- heme is held in position by a coordinating complex 245 (1949). between the central Fe(II) atom and 2 histidine residues 3. Radola, B.J., Isoelectric focusing in layers of (on helices E and F, respectively). -

(Glycosylated) Hemoglobin: Hba1c New Directions to Diagnose Diabetes
Article 368 1 Clock Hour Glycated (Glycosylated) Hemoglobin: HbA1c New directions to diagnose diabetes Joseph Balatbat 2nd Place Winner 2010 AMT Technical Writing Contest Also known as hemoglobin A1c, HbA1c, A1C or es the effectiveness of therapy by monitoring long- Hb1c, Glycated (Glycosylated) Hemoglobin is a term serum glucose regulation. In individuals with form of hemoglobin used primarily to identify the av- poorly controlled diabetes, the quantities of this erage plasma glucose concentration over a prolonged glycated hemoglobin are much higher than in period of time. Increased levels of glycated hemoglo- healthy people. bin has been associated with cardiovascular disease, Using the conversion table (See table 1) from the nephropathy, and retinopathy in diabetes mellitus. American Diabetes Association’s (ADA) 2005 posi- Monitoring the level of HbA1c in juvenile onset (type tion statement on Standards of Medical Care in Dia- 1– autoimmune) diabetes may improve treatment.1 betes, the 7.5% A1C reading would equate to an aver- age blood glucose of about 168mg/dL. Bear in mind Background that the correlation between mean plasma glucose lev- In 1958, hemoglobin A1C was first separated els and A1C levels is an estimation only, dependent on from other forms of hemoglobin (Huisman and Me- methodology used for the calculation as well as other tering) using a chromatographic column.2 Ten years factors, such as the red blood cells’ life span. A 1 per- later, hemoclobin A1C was characterized as a glyco- cent change in an A1C result reflects a change of about protein (non-enzymatic attachment of glucose to pro- 30mg/dL (1.67 mmol/L) in average blood glucose. -

Tests of Glycemia in Diabetes
POSITION STATEMENT Tests of Glycemia in Diabetes AMERICAN DIABETES ASSOCIATION onitoring of glycemic status, as which provide a comprehensive review of to increasing use of SMBG include cost performed by patients and health the subject (3,4). of testing, inadequate understanding by M care providers, is considered a both health care providers and patients cornerstone of diabetes care. Results of Recommendations about the health benefits and proper use monitoring are used to assess the efficacy 1. Based principally on the DCCT results, it of SMBG results, patient psychological of therapy and to guide adjustments in is recommended that most individuals and physical discomfort associated with medical nutrition therapy (MNT), exer- with diabetes should attempt to achieve finger-prick blood sampling, and incon- cise, and medications to achieve the best and maintain blood glucose levels as venience of testing in terms of time possible blood glucose control. close to normal as is safely possible. Be- requirements, physical setting, and This position statement presents the cause most patients with type 1 diabetes complexity of the technique. recommendations of the American Diabe- can achieve this goal only by using Given the importance of SMBG to di- tes Association on the tests used most SMBG, all treatment programs should abetes care, government, third-party widely in monitoring the glycemic status encourage SMBG for routine daily mon- payers, and others should strive to make of people with diabetes and addresses itoring. Daily SMBG is especially impor- the procedure readily accessible and af- both patient and physician/laboratory- tant for patients treated with insulin or fordable for all patients who require it. -
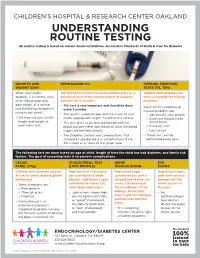
UNDERSTANDING ROUTINE TESTING All Routine Testing Is Based on Current American Diabetes Association Standards of Medical Care for Diabetes
CHILDREN’S HOSPITAL & RESEARCH CENTER OAKLAND UNDERSTANDING ROUTINE TESTING All routine testing is based on current American Diabetes Association Standards of Medical Care for Diabetes GROWTH AND HEMOGLOBIN A1c THYROID FUNCTION WEIGHT GAIN TESTS (T4, TSH) When your child’s This blood test is the measurement that gives us a Children with diabetes are diabetes is in control, your general picture of the overall control of diabetes more susceptible to thyroid child should grow and over the last 3 months. problems. gain weight at a normal • This test is very important and should be done Some of the symptoms of rate (following the growth every 3 months. thyroid problems are: curve in our chart). • The result is a percentage, and the closer to your » Abnormally slow growth • We measure your child’s child’s appropriate target, the better the control. » Excessive fatigue (tired height and weight at • This test gives us an idea (combined with the all the time) each clinic visit. blood glucose meter download) of what the blood » Extra dry skin sugars are between checks. » Loss of hair • The Diabetes Control and Complications Trial • These tests will be showed a huge decrease in complications if the performed every year. A1c is kept at or close to the target level. The following test are done based on age of child, length of time the child has had diabetes, and family risk factors. The goal of screening tests is to prevent complications. CELIAC CHOLESTEROL TEST URINE EYE PANEL (TTg) (LIPID PROFILE) MICROALBUMIN EXAMS Children with diabetes may be High levels of cholesterol High blood sugar High blood sugars at risk for celiac disease/gluten can contribute to heart concentrations over a over time can also intolerance. -

A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders
medicines Review A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders Andronicos Yiannikourides 1 and Gladys O. Latunde-Dada 2,* 1 Faculty of Life Sciences and Medicine, Henriette Raphael House Guy’s Campus King’s College London, London SE1 1UL, UK 2 Department of Nutritional Sciences, School of Life Course Sciences, King’s College London, Franklin-Wilkins-Building, 150 Stamford Street, London SE1 9NH, UK * Correspondence: [email protected] Received: 30 June 2019; Accepted: 2 August 2019; Published: 5 August 2019 Abstract: Iron is a vital trace element for humans, as it plays a crucial role in oxygen transport, oxidative metabolism, cellular proliferation, and many catalytic reactions. To be beneficial, the amount of iron in the human body needs to be maintained within the ideal range. Iron metabolism is one of the most complex processes involving many organs and tissues, the interaction of which is critical for iron homeostasis. No active mechanism for iron excretion exists. Therefore, the amount of iron absorbed by the intestine is tightly controlled to balance the daily losses. The bone marrow is the prime iron consumer in the body, being the site for erythropoiesis, while the reticuloendothelial system is responsible for iron recycling through erythrocyte phagocytosis. The liver has important synthetic, storing, and regulatory functions in iron homeostasis. Among the numerous proteins involved in iron metabolism, hepcidin is a liver-derived peptide hormone, which is the master regulator of iron metabolism. This hormone acts in many target tissues and regulates systemic iron levels through a negative feedback mechanism. Hepcidin synthesis is controlled by several factors such as iron levels, anaemia, infection, inflammation, and erythropoietic activity. -

Fructosamine Is a Valuable Marker for Glycemic Control And
www.nature.com/scientificreports OPEN Fructosamine is a valuable marker for glycemic control and predicting adverse outcomes following total hip arthroplasty: a prospective multi‑institutional investigation Noam Shohat1,2, Karan Goswami1, Leigham Breckenridge1, Michael B. Held3, Arthur L. Malkani4, Roshan P. Shah3, Ran Schwarzkopf5 & Javad Parvizi1* Recently, fructosamine has shown promising results in predicting adverse outcomes following total knee arthroplasty. The purpose of this study was to assess the utility of fructosamine to predict adverse outcomes following total hip arthroplasty (THA). A prospective multi‑center study involving four institutions was conducted. All primary THA were evaluated for glycemic control using fructosamine levels prior to surgery. Adverse outcomes were assessed at a minimum 1 year from surgery. Primary outcome of interest was periprosthetic joint infection (PJI) based on the International Consensus Meeting (ICM) criteria. Secondary outcomes assessed were superfcial infections, readmissions and death. Based on previous studies on the subject, fructosamine levels above 293 µmol/L were used to defne inadequate glycemic control. Overall 1212 patients were enrolled in the present study and were available for follow up at a minimum 1 year from surgery. Of those, 54 patients (4.5%) had elevated fructosamine levels (> 293 µmol/L) and these patients were 6.7 times more likely to develop PJI compared to patients with fructosamine levels below 293 µmol/L (p = 0.002). Patients with elevated fructosamine were also associated with more readmissions (16.7% vs. 4.4%, p < 0.007) and a higher mortality rate (3.7% vs. 0.6%, p = 0.057). These associations remained statistically signifcant in a multi‑regression analysis after adjusting for age, comorbidities and length of stay; Adjusted odds ratio were 6.37 (95% confdence interval 1.98–20.49, p = 0.002) for PJI and 2.68 (95% confdence interval 1.14–6.29, p = 0.023) for readmissions.