Detecting Type 2 Diabetes and Impaired Glucose Regulation Using Glycated Hemoglobin in Different Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WELLNESS SCREENING TEST EXPLANATION SHEET We Encourage You to Share All Lab Results with Your Primary Care Provider
WELLNESS SCREENING TEST EXPLANATION SHEET We encourage you to share all lab results with your primary care provider. EXPLANATION OF BLOOD TESTS HEMOGLOBIN A1C: A Hemoglobin A1c level is HEMATOLOGY WELLNESS: expressed as a percent and is used to evaluate the Blood counts are often used as a broad screening test average amount of glucose in the blood over the last to determine an individual’s general health status. 2 to 3 months. This test may be used to screen for and White blood cells are the cells that are part of the diagnose diabetes or risk of developing diabetes. body’s defense system against infections and cancer and also play a role in allergies and inflammation. ALT: This test is used to determine if a patient has Red blood cells are the cells that transport oxygen liver damage. In healthy individuals, ALT levels in the throughout the body. Hemoglobin measures the blood are low and they rise in various disease states. total amount of the oxygen-carrying protein in the blood, which generally reflects the number of red CALCIUM: A calcium test is ordered to screen for, blood cells in the blood. Hematocrit measures the diagnose and monitor a range of conditions percentage of a person’s total blood volume that relating to the bones, heart, nerves, kidneys and consists of red blood cells. Platelets are cell fragments teeth. The test may also be ordered if a person has that are vital for normal blood clotting. symptoms of parathyroid disorder, malabsorption or an overactive thyroid. FREE T4: Free T4 is used to help evaluate thyroid function, generally after determining the TSH is CREATININE: The creatinine blood test is used to abnormal. -

Genetically Determined Hypoalbuminemia As a Risk Factor for Hypertension: Instrumental Variable Analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2*
www.nature.com/scientificreports OPEN Genetically determined hypoalbuminemia as a risk factor for hypertension: instrumental variable analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2* Hypoalbuminemia is associated with vascular endothelial dysfunction and the development of chronic cardiovascular diseases. However, the relationship between serum albumin concentration and blood pressure changes remains controversial. Community‑based longitudinal cohort data collected from Korean Genome and Epidemiology Study were used in this study. Hypoalbuminemia was defned as a serum albumin concentration of ≤ 4.0 g/dL. A total of 4325 participants were categorized into control (n = 3157) and hypoalbuminemia (n = 1168) groups. Serum albumin had a non‑linear relationship with the risk of hypertension development. A genome‑wide association study revealed 71 susceptibility loci associated with hypoalbuminemia. Among susceptibility loci, genetic variations at rs2894536 in LOC107986598 and rs10972486 in ATP8B5P were related to elevated blood pressure. Serum albumin (HR = 0.654, 95% CI 0.521–0.820) and polymorphisms of rs2894536 (HR = 1.176, 95% CI 1.015–1.361) and rs10972486 (HR = 1.152, 95% CI 1.009–1.316) were signifcant predictors of hypertension development. Increased albumin concentration instrumented by 2 hypoalbuminemia‑associated SNPs (rs2894536 and rs10972486) was associated with decreased HRs for hypertension development (HR = 0.762, 95% CI 0.659–0.882 and HR = 0.759, 95% CI 0.656–0.878). Our study demonstrated that genetically determined hypoalbuminemia is a signifcant predictor of incipient hypertension. Albumin, one of the major serum proteins, has multiple important physiological functions involving stabilization of plasma colloid osmotic pressure, transportation of diverse substances, and signifcant antioxidant activity, and its concentration is fnely regulated by various systems in the physiologic state 1. -

Glycated Hemoglobin and Glycated Albumin in Patients with Diabetes
Kitajima et al. Renal Replacement Therapy (2020) 6:10 https://doi.org/10.1186/s41100-020-0260-5 RESEARCH Open Access Glycated hemoglobin and glycated albumin in patients with diabetes undergoing hemodiafiltration Yukie Kitajima1*, Shunichiro Urabe2, Takashi Hosono2, Satoshi Yoshikawa3, Yuzuru Sato3 and Toru Hyodo2 Abstract Background: Online hemodiafiltration (OHDF), which results in high albumin leakage, is now widely used in Japan for dialysis, since the national insurance system began reimbursing its costs in 2012. Glycated albumin (GA) levels are affected by albumin leakage into effluent dialysate fluid. Therefore, GA levels in patients requiring diabetes- related dialysis undergoing OHDF require monitoring. However, there have been no previous reports on glycemic control indicators of patients with diabetes undergoing OHDF. We aimed to develop a glycemic control index for patients requiring diabetes-related dialysis undergoing OHDF. Methods: This study comprised 133 diabetic patients undergoing OHDF. We examined the correlation between GA and glycated hemoglobin (HbA1c) levels. We analyzed effluent dialysate fluid samples from 41 patients classified into 3 groups, namely, group A, non-protein-leaking OHDF (n = 20); group B, protein-leaking OHDF (n = 14); and group C, highly efficient protein-leaking OHDF (n = 7). We examined the association between GA and HbA1c levels in each group and among patients. Results: A significant positive correlation was observed between GA and HbA1c levels (r = 0.562, p < 0.0001). There was no significant correlation between pre-dialysis blood glucose levels and HbA1c or GA levels as observed on regular blood tests performed under non-fasting conditions. Patients were classified into 2 groups based on their mean albumin levels (3.4 g/dL cutoff). -

Diagnosis of Insulinoma in a Maine Coon
JOR0010.1177/2055116919894782Journal of Feline Medicine and Surgery Open ReportsGifford et al 894782research-article2020 Case Report Journal of Feline Medicine and Surgery Open Reports Diagnosis of insulinoma in 1 –10 © The Author(s) 2020 Article reuse guidelines: a Maine Coon cat sagepub.com/journals-permissions DOI:https://doi.org/10.1177/2055116919894782 10.1177/2055116919894782 journals.sagepub.com/home/jfmsopenreports This paper was handled and processed by the American Editorial Office (AAFP) 1 2 3 Carol H Gifford , Anita P Morris , Kurt J Kenney and for publication in JFMS Open Reports J Scot Estep4 Abstract Case summary A 9-year-old male neutered Maine Coon cat presented with a 6-month history of polyphagia and one recent episode of tremors and weakness. Blood work revealed profound hypoglycemia and results of a paired insulin glucose test were consistent with an insulinoma. Abdominal ultrasound revealed a solitary pancreatic mass, and results of a fine-needle aspirate (FNA) gave further support for the location of the neuroendocrine tumor. After unsuccessful medical management of the hypoglycemia, the mass was surgically removed. Immunohistochemistry confirmed that it was an insulinoma. At the time of writing, the patient had been in clinical remission for 9 months. Relevance and novel information Feline insulinomas are rare and there is very little information on their behavior, clinical course and histologic characteristics. This is the first reported case of an insulinoma in a Maine Coon cat and the first to describe results of an ultrasound-guided FNA of the mass. In addition, the progression of disease, histopathology and immunohistochemistry results add to the currently minimal database for feline insulinomas. -

Levels of Serum Uric Acid in Patients with Impaired Glucose Tolerance
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 08, ISSUE 02, 2021 LEVELS OF SERUM URIC ACID IN PATIENTS WITH IMPAIRED GLUCOSE TOLERANCE 1,2Shahzad Ali Jiskani*, 1Lubna Naz, 3Ali Akbar Pirzado, 4Dolat Singh, 5Asadullah Hanif, 6Razia Bano 1Department of Pathology, Chandka Medical College, SMBBMU, Larkano 2Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan 3Department of Community Medicine and Public Health, Chadka Medical College, SMBBMU, Larkana 4Department of Medicine, Indus Medical College Hospital, Tando Muhammad Khan 5Health Department, Govt. of Sindh 6Department of Medicine, Liaquat University of Medical & Health Sciences Jamshoro *Corresponding Author: Shahzad Ali Jiskani Department of Pathology, Chandka Medical College, SMBBMU, Larkano ABSTRACT Objective: The main objective of this study is to see levels of serum uric acid in patients having impaired glucose tolerance as compared to normal glucose tolerant individuals. Patients and Methods: This was prospective study, conducted at Department of Medicine and Department of Pathology, Indus Medical College Hospital, Tando Muhammad Khan for the period of 8 months (March 2019 to October 2019). A total of 140 patients were included in this study. An oral glucose test was performed with load of 75g glucose in patients having impaired glucose in fasting. Both normal glucose tolerance and impaired glucose tolerance individuals were evaluated for their serum uric acid level. Serum glucose levels and serum uric acid levels were performed on COBAS C-111 Chemistry Analyzer ensuring procedures of quality control. Data was entered and analyzed using SPSS 21.0. Results: In patients having impaired glucose tolerance, the frequency of hyperuricemia was 62.35%. Analysis of both groups (patients with normal glucose tolerance and glucose intolerance), association was strong with p-value of <0.001. -

C-Reactive Protein and Gamma-Glutamyltransferase
Hindawi Publishing Corporation Experimental Diabetes Research Volume 2010, Article ID 761715, 8 pages doi:10.1155/2010/761715 Clinical Study C-Reactive Protein and Gamma-Glutamyltransferase Concentrations in Relation to the Prevalence of Type 2 Diabetes Diagnosed by Glucose or HbA1c Criteria in Chinese Adults in Qingdao, China J. Ren,1 Z. C. Pang,2 W. G. Gao,3, 4, 5 H. R. Nan,6 S. J. Wang,2 L. Zhang,3, 4, 5 and Q. Qiao3, 4 1 Department of Epidemiology and Health Statistics, Shandong University, Jinan 250012, China 2 Department of Non-Communicable Disease Prevention, Qingdao Municipal Centre for Disease Control and Prevention, no. 175, Shandong Road, Qingdao 266071, China 3 Department of Public Health, University of Helsinki, 00014 Helsinki, Finland 4 Diabetes Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, 00014 Helsinki, Finland 5 Qingdao Endocrinology and Diabetes Hospital, 266071 Qingdao, China 6 Hong Kong Institute of Diabetes and Obesity, Hong Kong, China CorrespondenceshouldbeaddressedtoZ.C.Pang,[email protected] Received 13 August 2010; Revised 12 October 2010; Accepted 12 October 2010 Academic Editor: Giuseppe Paolisso Copyright © 2010 J. Ren et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aims. To investigate the association of C-reactive protein (CRP) and gamma glutamyltransferase (GGT) concentrations with newly diagnosed diabetes defined by either glucose or HbA1c criteria in Chinese adults. Methods. A population-based cross-sectional study was conducted in 2006. Data from 1167 men and 1607 women aged 35–74 years were analyzed. -

Profiling Glycated Hemoglobin Level, Lactate Dehydrogenase And
International Journal of Medical Laboratory 2017;4(2):135-141. Original Article Profiling Glycated Hemoglobin Level, Lactate Dehydrogenase and Alkaline Phosphatase Activity in Gestational Diabetes Mellitus Obese Women and Compare Them with Each Other Mohammadreza Nadimi Barforoushi1M.Sc. , Durdi Qujeq2,3*Ph.D Bostan Roudi1Ph.D. 1Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran. 2Department of Clinical Biochemistry, Faculty of Medicine, Babol University of Medical Sciences, Babol, Iran. 3Cellular and Molecular Biology Research Center (CMBRC), Health Research Institute, Babol University of Medical Sciences, Babol, Iran. A B S T R A C T Article history Background and Aims: The aim of this study was profiling glycated Received 16 Feb 2017 hemoglobin (HbA1c) level, lactate dehydrogenase (LDH) and alkaline Accepted 7 May 2017 phosphatase (ALP) activity in obese women with gestational diabetes Available online 28 Jun 2017 mellitus (GDM) and evaluating the correlation between them. Key words Materials and Methods: Sample size was 90 subjects admitted to the Alkaline phosphatase activity clinical laboratory, who were divided into three groups, in each group Gestational diabetes mellitus (n=30). Subjects glycemic control was checked by HbA1c; ALP, LDH Glycated hemoglobin activity and serum glucose were determined with commercial kit. Age and Lactate dehydrogenase body mass index (BMI) was recorded for each subject. The correlation analysis between blood activity of ALP, LDH activity, HbA1c, glucose, BMI and age in diabetic and normal pregnant women was carried out. Results: The mean of HbA1c level was significantly higher in the GDM obese women than in women with normal pregnancy (p=0.01). In contrast, the means of ALP and LDH activity were lower in the GDM obese women Downloaded from ijml.ssu.ac.ir at 3:56 IRST on Thursday September 30th 2021 than in women with normal pregnancy (p=0.09, and p=0.15, respectively). -
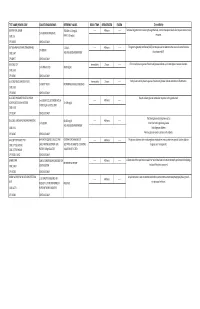
G's- Lab Test Menu
TEST NAME/ PSYCHE CODE COLLECTION GUIDELINES REFERENCE VALUES ROOM TEMP. REFRIGERATED FROZEN Clinical Utility GENTAMICIN, SERUM TROUGH: 0-2.0 mg/dL ------ 48 hours ------ Tests level of gentamicin circulating through the body. Certain therapeutic levels elicit require anti-microbial 2 ml SERUM. REFRIGERATE. CODE: GE PEAK: 5-10 mg/dL response. CPT: 81070 SCHEDULE: DAILY GGT (GAMMA GLUTAMYL TRANSFERASE) 5-31 IU/L ------ 48 hours ------ The gamma-glutamyl transferase (GGT) test may be used to determine the cause of elevated alkaline 1ml SERUM CODE: GGT AGE AND GENDER DEPENDENT phosphatase (ALP). CPT:82977 SCHEDULE: DAILY GLUCOSE, CSF Immediately 2 hours ------ CFS normally has no glucose. Presence of glucose indicates spinal meningitis or seizure disorders. 1 ml SPINAL FLUID 40-80 mg/mL CODE: GLFL CPT: 82947 SCHEDULE: DAILY GLUCOSE, MISCELLANEOUS FLUID Immediately 2 hours ------ Body fluids normally have no glucose. Presence of glucose indicates infection or inflammation. 1 ml BODY FLUID NO NORMAL RANGE ESTABLISHED CODE: GLFL CPT: 82945 SCHEDULE: DAILY GLUCOSE, PREGNANT RELATED 1 HOUR Results indicate glucose metabolism response to the glucola load. 1 ml SERUM COLLECTED ONE HOUR ------ 48 hours ------ LOADING (O'SULLIVAN SCREEN) 70-139 mg/dL AFTER 50 gm GLUCOSE LOAD CODE: GESC CPT: 82947 SCHEDULE: DAILY The blood glucose test may be used to: GLUCOSE, SERUM (FASTING AND RANDOM) 60-115 mg/dL ------ 48 hours ------ 1 ml SERUM Screen for both high blood glucose AGE AND GENDER DEPENDENT CODE: GLU Help diagnose diabetes CPT: 82947 SCHEDULE: DAILY Monitor glucose levels in persons with diabetes GLUCOSE TOLERANCE TEST 8HR FAST REQUIRED. COLLECT AND CRITERIA FOR DIAGNOSIS OF ------ 48 hours ------ The glucose tolerance test monitors glucose metabolism over a certain time period. -

To Study the Co-Relationship Between Glycosylated Hemoglobin and Serum Calcium Levels in Type 2 Diabetes Mellitus Patients
International Journal of Medical and Health Research International Journal of Medical and Health Research ISSN: 2454-9142 Received: 13-01-2020; Accepted: 14-02-2020; Published: 09-03-2020 www.medicalsciencejournal.com Volume 6; Issue 03; 2020; Page No. 43-45 To study the co-relationship between glycosylated hemoglobin and serum calcium levels in type 2 diabetes mellitus patients Dr. Hardeep Singh Deep1, Dr. Jasmine Kaur2, Dr. Gurjyot Singh Nanda3, Dr. Seerat Kaur4 1 Professor MD Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 2 Assistant. Professor MD Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 3 junior resident Medicine Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India 4 junior resident Radiodiagnosis Sri Guru Ram Das University of Health Sciences, Amritsar, Punjab, India Abstract Background: The incidence of type-2 Diabetes Mellitus has increased world-wide making it a major public health problem. Electrolyte and mineral abnormalities are common in patients with type-2 Diabetes Mellitus. Therefore, this study was undertaken to look for the correlation between HbA1c (glycated hemoglobin) and serum calcium levels in patients with type-2 Diabetes Mellitus. Aim: To study the co-relationship between glycosylated hemoglobin and serum calcium levels in type 2 diabetes mellitus patients. Materials and Methods: A total of 50 type 2 Diabetic patients and 50 healthy non-Diabetic individuals were included for the study. Both fasting and post prandial blood samples were collected from the two groups and were used for fasting blood sugar, HbA1c, serum calcium, RFT, LFT, CBC, UACR. -

Correlation Between Glycated Hemoglobin and Venous Blood Sugar in Diabetic Patients Monitored in Abidjan
Vol. 14(4), pp. 135-141, October-December 2020 DOI: 10.5897/AJBR2020.1102 Article Number: CD65C6E65033 ISSN 1996-0778 Copyright © 2020 Author(s) retain the copyright of this article African Journal of Biochemistry Research http://www.academicjournals.org/AJBR Full Length Research Paper Correlation between glycated hemoglobin and venous blood sugar in diabetic patients monitored in Abidjan MONDE Aké Absalome1*, CAMARA-CISSE Massara2, KOFFI Konan Gervais2, DIALLO Issiagha3, AKE AKE Alexandre4, YAPO-AKE Bénédicte4, ECRABEY Yann Christian3, KOUAKOU Francisk3, BENE YAO Roger Espérance4 and TIAHOU Georges5 1Félix HOUPHOUËT-BOIGNY University, Cocody, Abidjan, Côte d’Ivoire. 2Biochemistry Laboratory, Abidjan Medical School, Félix HOUPHOUËT BOIGNY University, Côte D'ivoire. 3Biochemistry Laboratory, University Hospital Center of Treichville, Côte D'ivoire. 4Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Félix HOUPHOUËT-BOIGNY University, Côte D'ivoire. 5Laboratory of Medical Biochemistry, Faculty of Medical Sciences, Alassane OUATTARA University, Bouaké, Côte D'ivoire. Received 23 August, 2020; Accepted 2 October, 2020 The aim of this study was to determine the correlation between glycated hemoglobin and blood sugar levels in diabetic subjects carried out in Abidjan. This cross-sectional study included 100 patients with diabetes monitored, for three months, for whom glycated blood glucose and hemoglobin were performed, this after informed consent of the patients. Pearson and Spearman correlation tests were used, at the 5% threshold. The patients with normal HbA1C and normal blood glucose accounted for 55.34 and 32%, respectively. A sedentary lifestyle and body mass index > 25 kg/m² were associated with a significant increase in the risk of increased blood glucose and HbA1C. -

(Glycosylated) Hemoglobin: Hba1c New Directions to Diagnose Diabetes
Article 368 1 Clock Hour Glycated (Glycosylated) Hemoglobin: HbA1c New directions to diagnose diabetes Joseph Balatbat 2nd Place Winner 2010 AMT Technical Writing Contest Also known as hemoglobin A1c, HbA1c, A1C or es the effectiveness of therapy by monitoring long- Hb1c, Glycated (Glycosylated) Hemoglobin is a term serum glucose regulation. In individuals with form of hemoglobin used primarily to identify the av- poorly controlled diabetes, the quantities of this erage plasma glucose concentration over a prolonged glycated hemoglobin are much higher than in period of time. Increased levels of glycated hemoglo- healthy people. bin has been associated with cardiovascular disease, Using the conversion table (See table 1) from the nephropathy, and retinopathy in diabetes mellitus. American Diabetes Association’s (ADA) 2005 posi- Monitoring the level of HbA1c in juvenile onset (type tion statement on Standards of Medical Care in Dia- 1– autoimmune) diabetes may improve treatment.1 betes, the 7.5% A1C reading would equate to an aver- age blood glucose of about 168mg/dL. Bear in mind Background that the correlation between mean plasma glucose lev- In 1958, hemoglobin A1C was first separated els and A1C levels is an estimation only, dependent on from other forms of hemoglobin (Huisman and Me- methodology used for the calculation as well as other tering) using a chromatographic column.2 Ten years factors, such as the red blood cells’ life span. A 1 per- later, hemoclobin A1C was characterized as a glyco- cent change in an A1C result reflects a change of about protein (non-enzymatic attachment of glucose to pro- 30mg/dL (1.67 mmol/L) in average blood glucose. -

Clinical Performance of Flash Glucose Monitoring System in Patients With
www.nature.com/scientificreports OPEN Clinical Performance of Flash Glucose Monitoring System in Patients with Liver Cirrhosis and Diabetes Mellitus Dalila Costa1,2 ✉ , Joana Lourenço1, Ana Margarida Monteiro3, Beatriz Castro1, Patricia Oliveira3, Maria Carmo Tinoco3, Vera Fernandes3, Olinda Marques3, Raquel Gonçalves2 & Carla Rolanda1,2 Flash glucose monitoring system (FGMS) is an improved subset of continuous glucose monitoring with a recognized efectiveness on glycemic control, though validation in patients with Liver Cirrhosis (LC) is lacking. To evaluate the accuracy of FGMS in patients with Type 2 Diabetes Mellitus (DM) and LC, a prospective, case-control study was performed in 61 ambulatory patients with LC and DM (LC group, n = 31) or DM (Control group, n = 30). During 14 days, patients performed 4 assessments per day of self-monitoring of blood glucose (SMBG, reference value) followed by FGMS scanning. There were 2567 paired SMBG and FGMS values used in the accuracy analysis, with an overall mean absolute relative diference (MARD) of 12.68% in the LC group and 10.55% in the control group (p < 0,001). In patients with LC, the percentage of readings within Consensus Consensus Error Grid analysis Zone A and A + B were 80.36% and 99,26%, respectively. Sensor clinical accuracy was not afected by factors such as body mass index, age, gender, Child-Pugh score or edematoascitic decompensation. This is the frst study to approach FGMS clinical accuracy in LC, revealing a potential usability of this system to monitor glycemic control in this population. Type 2 Diabetes Mellitus (DM) has been considered a Public Health challenge due to its signifcant impact on mortality and morbidity worldwide1.