Effects of Embryonic Responses to Clutch Mates on Egg Hatching Title Patterns of Pentatomidae (Heteroptera)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jordan Beans RA RMO Dir
Importation of Fresh Beans (Phaseolus vulgaris L.), Shelled or in Pods, from Jordan into the Continental United States A Qualitative, Pathway-Initiated Risk Assessment February 14, 2011 Version 2 Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology United States Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Pest Risk Assessment for Beans from Jordan Executive Summary In this risk assessment we examined the risks associated with the importation of fresh beans (Phaseolus vulgaris L.), in pods (French, green, snap, and string beans) or shelled, from the Kingdom of Jordan into the continental United States. We developed a list of pests associated with beans (in any country) that occur in Jordan on any host based on scientific literature, previous commodity risk assessments, records of intercepted pests at ports-of-entry, and information from experts on bean production. This is a qualitative risk assessment, as we express estimates of risk in descriptive terms (High, Medium, and Low) rather than numerically in probabilities or frequencies. We identified seven quarantine pests likely to follow the pathway of introduction. We estimated Consequences of Introduction by assessing five elements that reflect the biology and ecology of the pests: climate-host interaction, host range, dispersal potential, economic impact, and environmental impact. We estimated Likelihood of Introduction values by considering both the quantity of the commodity imported annually and the potential for pest introduction and establishment. We summed the Consequences of Introduction and Likelihood of Introduction values to estimate overall Pest Risk Potentials, which describe risk in the absence of mitigation. -

Die Raupenfliegen (Diptera: Tachinidae) Mitteleuropas: Bestimmungstabellen Und Angaben Zur Verbreitung Und Ökologie Der Einzelnen Arten
5 download Biodiversity Heritage Library, http://www.biodiversitylibrary.org/ Stuttgarter Beiträge zur Naturkunde Serie A (Biologie) Herausgeber: 4fr für Naturkunde, RosensteinV 70 19 l; , Staatliches Museum 1, D- r Stuttgart Stuttgarter Beitr. Naturk. Ser. A Nr. 506 170 S ,4. 9. 19.94 Professor Dr. Bernhard Ziegler zum 65. Geburtstag Die Raupenfliegen (Diptera: Tachinidae) Mitteleuropas: Bestimmungstabellen und Angaben zur Verbreitung und Ökologie der einzelnen Arten The Tachinids (Diptera: Tachinidae) of Central Europe: Identification Keys for the Species and Data on Distribution and Ecology Von Hans-Peter Tschorsnig und Benno Herting, Stuttgart Mit 291 Abbildungen Summary Keys are given for all central and northern European species of Tachinidae (Diptera). The most important data on distribution and ecology (mainly habitat, phenology, and host-range) are listed for the central European species. Zuammenfassung Es werden Bestimmungsschlüssel für alle in Mittel- und Nordeuropa vorkommenden Arten der Tachinidae (Diptera) gegeben. Für jede mitteleuropäische Art werden die wichtigsten Kenndaten zur Verbreitung und Ökologie (vor allem Habitat, Flugzeit und Wirtskreis) aufge- listet. Inhalt 1. Einleitung 2 2. Beg'iff.c erklärungen 4 2.1. Allgemeines 4 2.2. Kopf 5 2.3. Thorax 7 2.4. Flügel 8 2.5. Beine 9 2.6. Abdomen 10 2.7. Bereifung 11 2.8. Färbung . 11 download Biodiversity Heritage Library, http://www.biodiversitylibrary.org/ 2 STUTTGARTER BEITRÄGE ZUR NATURKUNDE Ser. A, Nr. 506 2.9. Körpergröße 11 2.10. Abkürzungen 11 3. Schlüssel für die Gattungen 12 4. Schlüssel für die Arten . 42 4.1. Subfamilie Exoristinae 42 4.2. Subfamilie Tachininae 63 4.3. Subfamilie Dexiinae 79 4.4. -

Download the Survey Results
WILDLIFE IN COMMON SURVEY Wildlife in Common Survey Site Name: Pit Common (also known as Drove Hill Common) Parish: Southrepps Grid reference: TG 262353 Area: 0.36 hectares District: North Norfolk Survey date: 17 September 2018 Registered Common Number – CL 390 Pond on Pit Common 1 WILDLIFE IN COMMON SURVEY Annotated habitat map (showing target note numbers): 2 WILDLIFE IN COMMON SURVEY Habitat map 3 WILDLIFE IN COMMON SURVEY Habitat description: Situated in Lower Street Southrepps, Pit Common (registered as Drove Hill Common) is a small area of pond, grassland, scrub and planted trees, which rises up away from the road. Number Target Note (see map) 1 G1 (pond), F2.1 (Marginal vegetation) & A2 (Willow scrub) Pond. At the time of the survey the pond was dry. Invaded with New Zealand pygmy weed (Crassula helmsii) to the south and greater reed mace (Typha latifolia) to the North with a stand of common reed (Phragmites australis) to the East there was little open water. Small patches of reed canary grass (Phalaris arundinacea) to the east and west banks. Coppiced willow (Salix sp.) to the Northern end. Previous management of the pond has resulted in the presence of water lilies (Nyphaea alba). Also present are water plantain (Alisma plantago-aquatica), celery-leaved buttercup (Ranunculus scleratus), soft rush (Juncus effusus), hard rush (Juncus inflexus), and galingale (Cyperus longus). In 2009 the pond was completely dredged and a large area of open water formed. This has slowly silted up again and become overrun with the bulrush (Typha latifolia) and the Crassula that appeared this year (2018). -

Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs
20182018 Green RoofsUrban and Naturalist Urban Biodiversity SpecialSpecial Issue No. Issue 1:16–38 No. 1 A. Nagase, Y. Yamada, T. Aoki, and M. Nomura URBAN NATURALIST Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs Ayako Nagase1,*, Yoriyuki Yamada2, Tadataka Aoki2, and Masashi Nomura3 Abstract - Urban biodiversity is an important ecological goal that drives green-roof in- stallation. We studied 2 kinds of green roofs designed to optimize biodiversity benefits: the Harappa (extensive) roof and the Biotope (intensive) roof. The Harappa roof mimics vacant-lot vegetation. It is relatively inexpensive, is made from recycled materials, and features community participation in the processes of design, construction, and mainte- nance. The Biotope roof includes mainly native and host plant species for arthropods, as well as water features and stones to create a wide range of habitats. This study is the first to showcase the Harappa roof and to compare biodiversity on Harappa and Biotope roofs. Arthropod species richness was significantly greater on the Biotope roof. The Harappa roof had dynamic seasonal changes in vegetation and mainly provided habitats for grassland fauna. In contrast, the Biotope roof provided stable habitats for various arthropods. Herein, we outline a set of testable hypotheses for future comparison of these different types of green roofs aimed at supporting urban biodiversity. Introduction Rapid urban growth and associated anthropogenic environmental change have been identified as major threats to biodiversity at a global scale (Grimm et al. 2008, Güneralp and Seto 2013). Green roofs can partially compensate for the loss of green areas by replacing impervious rooftop surfaces and thus, contribute to urban biodiversity (Brenneisen 2006). -

Bilimsel Araştırma Projesi (8.011Mb)
1 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) 2 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) ÖZET* 3 AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Yapılan bu çalışma ile Amasya, Sivas ve Tokat illerinin Kelkit havzasına ait kısımlarında bulunan ve farklı böcek takımlarında parazitoit olarak yaşayan Tachinidae (Diptera) türleri, bunların tanımları ve yayılışlarının ortaya konulması amaçlanmıştır. Bunun için farklı böcek takımlarına ait türler laboratuvarda kültüre alınarak parazitoit olarak yaşayan Tachinidae türleri elde edilmiştir. Kültüre alınan Lepidoptera takımına ait türler içerisinden, Euproctis chrysorrhoea (L.), Lymantria dispar (L.), Malacosoma neustrium (L.), Smyra dentinosa Freyer, Thaumetopoea solitaria Freyer, Thaumetopoea sp. ve Vanessa sp.,'den parazitoit elde edilmiş, -

Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha Halys in Europe with a Large-Scale Monitoring Program
insects Article Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha halys in Europe with a Large-Scale Monitoring Program Livia Zapponi 1 , Francesco Tortorici 2 , Gianfranco Anfora 1,3 , Simone Bardella 4, Massimo Bariselli 5, Luca Benvenuto 6, Iris Bernardinelli 6, Alda Butturini 5, Stefano Caruso 7, Ruggero Colla 8, Elena Costi 9, Paolo Culatti 10, Emanuele Di Bella 9, Martina Falagiarda 11, Lucrezia Giovannini 12, Tim Haye 13 , Lara Maistrello 9 , Giorgio Malossini 6, Cristina Marazzi 14, Leonardo Marianelli 12 , Alberto Mele 15 , Lorenza Michelon 16, Silvia Teresa Moraglio 2 , Alberto Pozzebon 15 , Michele Preti 17 , Martino Salvetti 18, Davide Scaccini 15 , Silvia Schmidt 11, David Szalatnay 19, Pio Federico Roversi 12 , Luciana Tavella 2, Maria Grazia Tommasini 20, Giacomo Vaccari 7, Pietro Zandigiacomo 21 and Giuseppino Sabbatini-Peverieri 12,* 1 Centro Ricerca e Innovazione, Fondazione Edmund Mach (FEM), Via Mach 1, 38098 S. Michele all’Adige, TN, Italy; [email protected] (L.Z.); [email protected] (G.A.) 2 Dipartimento di Scienze Agrarie, Forestali e Alimentari, University di Torino (UniTO), Largo Paolo Braccini 2, 10095 Grugliasco, TO, Italy; [email protected] (F.T.); [email protected] (S.T.M.); [email protected] (L.T.) 3 Centro Agricoltura Alimenti Ambiente (C3A), Università di Trento, Via Mach 1, 38098 S. Michele all’Adige, TN, Italy 4 Fondazione per la Ricerca l’Innovazione e lo Sviluppo Tecnologico dell’Agricoltura Piemontese (AGRION), Via Falicetto 24, 12100 Manta, CN, -

Folk Taxonomy, Nomenclature, Medicinal and Other Uses, Folklore, and Nature Conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3
Ulicsni et al. Journal of Ethnobiology and Ethnomedicine (2016) 12:47 DOI 10.1186/s13002-016-0118-7 RESEARCH Open Access Folk knowledge of invertebrates in Central Europe - folk taxonomy, nomenclature, medicinal and other uses, folklore, and nature conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3 Abstract Background: There is scarce information about European folk knowledge of wild invertebrate fauna. We have documented such folk knowledge in three regions, in Romania, Slovakia and Croatia. We provide a list of folk taxa, and discuss folk biological classification and nomenclature, salient features, uses, related proverbs and sayings, and conservation. Methods: We collected data among Hungarian-speaking people practising small-scale, traditional agriculture. We studied “all” invertebrate species (species groups) potentially occurring in the vicinity of the settlements. We used photos, held semi-structured interviews, and conducted picture sorting. Results: We documented 208 invertebrate folk taxa. Many species were known which have, to our knowledge, no economic significance. 36 % of the species were known to at least half of the informants. Knowledge reliability was high, although informants were sometimes prone to exaggeration. 93 % of folk taxa had their own individual names, and 90 % of the taxa were embedded in the folk taxonomy. Twenty four species were of direct use to humans (4 medicinal, 5 consumed, 11 as bait, 2 as playthings). Completely new was the discovery that the honey stomachs of black-coloured carpenter bees (Xylocopa violacea, X. valga)were consumed. 30 taxa were associated with a proverb or used for weather forecasting, or predicting harvests. Conscious ideas about conserving invertebrates only occurred with a few taxa, but informants would generally refrain from harming firebugs (Pyrrhocoris apterus), field crickets (Gryllus campestris) and most butterflies. -

Key for the Separation of Halyomorpha Halys (Stål)
Key for the separation of Halyomorpha halys (Stål) from similar-appearing pentatomids (Insecta : Heteroptera : Pentatomidae) occuring in Central Europe, with new Swiss records Autor(en): Wyniger, Denise / Kment, Petr Objekttyp: Article Zeitschrift: Mitteilungen der Schweizerischen Entomologischen Gesellschaft = Bulletin de la Société Entomologique Suisse = Journal of the Swiss Entomological Society Band (Jahr): 83 (2010) Heft 3-4 PDF erstellt am: 11.10.2021 Persistenter Link: http://doi.org/10.5169/seals-403015 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das -

Discrimination Between Eggs from Stink Bugs Species in Europe Using MALDI-TOF MS
insects Article Discrimination between Eggs from Stink Bugs Species in Europe Using MALDI-TOF MS Michael A. Reeve 1,* and Tim Haye 2 1 CABI, Bakeham Lane, Egham, Surrey TW20 9TY, UK 2 CABI, Rue des Grillons 1, CH-2800 Delémont, Switzerland; [email protected] * Correspondence: [email protected] Simple Summary: Recent globalization of trade and travel has led to the introduction of exotic insects into Europe, including the brown marmorated stink bug (Halyomorpha halys)—a highly polyphagous pest with more than 200 host plants, including many agricultural crops. Unfortunately, farmers and crop-protection advisers finding egg masses of stink bugs during crop scouting frequently struggle to identify correctly the species based on their egg masses, and easily confuse eggs of the invasive H. halys with those of other (native) species. To this end, we have investigated using matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) for rapid, fieldwork-compatible, and low-reagent-cost discrimination between the eggs of native and exotic stink bugs. Abstract: In the current paper, we used a method based on stink bug egg-protein immobilization on filter paper by drying, followed by post-(storage and shipping) extraction in acidified acetonitrile containing matrix, to discriminate between nine different species using MALDI-TOF MS. We obtained 87 correct species-identifications in 87 blind tests using this method. With further processing of the unblinded data, the highest average Bruker score for each tested species was that of the cognate Citation: Reeve, M.A.; Haye, T. reference species, and the observed differences in average Bruker scores were generally large and the Discrimination between Eggs from errors small except for Capocoris fuscispinus, Dolycoris baccarum, and Graphosoma italicum, where the Stink Bugs Species in Europe Using average scores were lower and the errors higher relative to the remaining comparisons. -
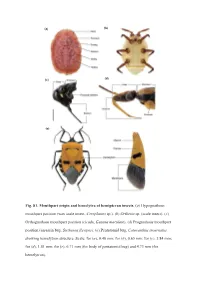
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

Invasive Stink Bugs and Related Species (Pentatomoidea) Biology, Higher Systematics, Semiochemistry, and Management
Invasive Stink Bugs and Related Species (Pentatomoidea) Biology, Higher Systematics, Semiochemistry, and Management Edited by J. E. McPherson Front Cover photographs, clockwise from the top left: Adult of Piezodorus guildinii (Westwood), Photograph by Ted C. MacRae; Adult of Murgantia histrionica (Hahn), Photograph by C. Scott Bundy; Adult of Halyomorpha halys (Stål), Photograph by George C. Hamilton; Adult of Bagrada hilaris (Burmeister), Photograph by C. Scott Bundy; Adult of Megacopta cribraria (F.), Photograph by J. E. Eger; Mating pair of Nezara viridula (L.), Photograph by Jesus F. Esquivel. Used with permission. All rights reserved. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2018 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed on acid-free paper International Standard Book Number-13: 978-1-4987-1508-9 (Hardback) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materi- als or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, micro- filming, and recording, or in any information storage or retrieval system, without written permission from the publishers. -

Diversity and Abundance of Phytophage Hemiptera in an Irrigated Rice Ecosystem of Tamil Nadu, India
Asian102 J. of Bio Sci. (April, 2008) Vol. 3 No. 1 : (102-106) Diversity and abundance of phytophage hemiptera in an irrigated rice ecosystem of Tamil Nadu, India M. KANDIBANE Department of Agricultural Entomology and Plant Nematology, Pandit Jawaharlal Nehru College of Agriculture and Research Institute, KARAIKAL (PUDUCHERRY) INDIA. (Accepted : January, 2008) A total of 24 taxa of phytophage hemipterans were recorded in weeded and partially weeded rice ecosystems during kharif 2001.Among them, thirteen taxa viz., Brevennia rehi (Lindinger), Hysteroneura setariae (Thomas), Nilaparvata lugens (Stal), Nepotettix virescens (Distant), Cofana spectra (Distant), Recilia dorsalis (Motsch.), Sogatella furcifera (Horvath), Nezara viridula (Lin.), Dolycoris indicus (Stal.), Menida histrio Fab., Scotinophara lurida (Burmeister), Leptocorisa acuta (Thunberg) and L. oratorius (Fabricius) showed greater abundance in weeded rice ecosystem, while rest of the species of phytophage hemipterans exhibited more abundance only in partially weeded plots. The diversity of delphacids had perfect similarity through out the season. During tillering and flowering stages of the crop, cicadellids, pentatomids and red spotted bug exhibited greater diversity (= less similarity). Earhead bugs showed higher diversity during flowering and dough stages. Aphid exhibited perfect similarity in the first and second week, and was absent from the third week to seventh week. A Total of 18 weed species acted as alternate hosts for polyphagous phytophage hemipterans were recorded in partially weeded plot. Of them, Cyperus iria, C. diformis, C. rotundus, Echinochloa colonum, E. crus –galli, Ipomea aquatica and Marsilea quadrifolia were dominant. Key words: Diversity, similarity, relative abundance, weed plants, weeded rice ecosystem, partially weeded rice ecosystem, hemipteran insects. INTRODUCTION identified by the Zoological Survey of India, Calcutta as everal species of leafhoppers and planthoppers are Dolycoris indicus Stal and D.