Equation of State for Supercooled Water at Pressures up to 400 Mpa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physics, Chapter 17: the Phases of Matter
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 17: The Phases of Matter Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 17: The Phases of Matter" (1958). Robert Katz Publications. 165. https://digitalcommons.unl.edu/physicskatz/165 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 17 The Phases of Matter 17-1 Phases of a Substance A substance which has a definite chemical composition can exist in one or more phases, such as the vapor phase, the liquid phase, or the solid phase. When two or more such phases are in equilibrium at any given temperature and pressure, there are always surfaces of separation between the two phases. In the solid phase a pure substance generally exhibits a well-defined crystal structure in which the atoms or molecules of the substance are arranged in a repetitive lattice. Many substances are known to exist in several different solid phases at different conditions of temperature and pressure. These solid phases differ in their crystal structure. Thus ice is known to have six different solid phases, while sulphur has four different solid phases. -

Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please Show All Work for Credit
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1. 1 2. –1 A least squares fit of ln P versus 1/T gives the result Hvaporization = 25.28 kJ mol . 3. Assuming constant pressure and temperature, and that the surface area of the protein is reduced by 25% due to the hydrophobic interaction: 2 G 0.25 N A 4 r Convert to per mole, ↓determine size per molecule 4 r 3 V M N 0.73mL/ g 60000g / mol (6.02 1023) 3 2 2 A r 2.52 109 m 2 23 9 2 G 0.25 N A 4 r 0.0720N / m 0.25 6.02 10 / mol (4 ) (2.52 10 m) 865kJ / mol We think this is a reasonable approach, but the value seems high 2 4. The vapor pressure of an unknown solid is approximately given by ln(P/Torr) = 22.413 – 2035(K/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr) = 18.352 – 1736(K/T). a. Calculate Hvaporization and Hsublimation. b. Calculate Hfusion. c. Calculate the triple point temperature and pressure. a) Calculate Hvaporization and Hsublimation. From Equation (8.16) dPln H sublimation dT RT 2 dln P d ln P dT d ln P H T 2 sublimation 11 dT dT R dd TT For this specific case H sublimation 2035 H 16.92 103 J mol –1 R sublimation Following the same proedure as above, H vaporization 1736 H 14.43 103 J mol –1 R vaporization b. -

Phase Diagrams a Phase Diagram Is Used to Show the Relationship Between Temperature, Pressure and State of Matter
Phase Diagrams A phase diagram is used to show the relationship between temperature, pressure and state of matter. Before moving ahead, let us review some vocabulary and particle diagrams. States of Matter Solid: rigid, has definite volume and definite shape Liquid: flows, has definite volume, but takes the shape of the container Gas: flows, no definite volume or shape, shape and volume are determined by container Plasma: atoms are separated into nuclei (neutrons and protons) and electrons, no definite volume or shape Changes of States of Matter Freezing start as a liquid, end as a solid, slowing particle motion, forming more intermolecular bonds Melting start as a solid, end as a liquid, increasing particle motion, break some intermolecular bonds Condensation start as a gas, end as a liquid, decreasing particle motion, form intermolecular bonds Evaporation/Boiling/Vaporization start as a liquid, end as a gas, increasing particle motion, break intermolecular bonds Sublimation Starts as a solid, ends as a gas, increases particle speed, breaks intermolecular bonds Deposition Starts as a gas, ends as a solid, decreases particle speed, forms intermolecular bonds http://phet.colorado.edu/en/simulation/states- of-matter The flat sections on the graph are the points where a phase change is occurring. Both states of matter are present at the same time. In the flat sections, heat is being removed by the formation of intermolecular bonds. The flat points are phase changes. The heat added to the system are being used to break intermolecular bonds. PHASE DIAGRAMS Phase diagrams are used to show when a specific substance will change its state of matter (alignment of particles and distance between particles). -

CH 222 Oregon State University Week 6 Worksheet Notes
CH 222 Oregon State University Week 6 Worksheet Notes 1. Place the following compounds in order of decreasing strength of intermolecular forces. HF O2 CO2 HF > CO2 > O2 2. In liquid propanol, CH3CH2CH2OH, which intermolecular forces are present? Dispersion, hydrogen bonding and dipole-dipole forces are present. 3. Assign the appropriate labels to the phase diagram shown below. A) A = liquid, B = solid, C = gas, D = critical point B) A = gas, B = solid, C = liquid, D = triple point C) A = gas, B = liquid, C = solid, D = critical point D) A = solid, B = gas, C = liquid, D = supercritical fluid E) A = liquid, B = gas, C = solid, D = triple point 4. Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500°C, what phase changes will occur (in order of increasing temperature)? A) condensation, followed by vaporization B) sublimation, followed by deposition C) vaporization, followed by deposition D) fusion (melting), followed by vaporization E) No phase change will occur under the conditions specified. 5. Which of the following solutions will have the highest concentration of chloride ions? A) 0.10 M NaCl B) 0.10 M MgCl2 C) 0.10 M AlCl3 D) 0.05 M CaCl2 E) All of these solutions have the same concentration of chloride ions. 6. Commercial grade HCl solutions are typically 39.0% (by mass) HCl in water. Determine the molarity of the HCl, if the solution has a density of 1.20 g/mL. A) 7.79 M B) 10.7 M C) 12.8 M D) 9.35 M E) 13.9 M 7. -

Triple Point
AccessScience from McGraw-Hill Education Page 1 of 2 www.accessscience.com Triple point Contributed by: Robert L. Scott Publication year: 2014 A particular temperature and pressure at which three different phases of one substance can coexist in equilibrium. In common usage these three phases are normally solid, liquid, and gas, although triple points can also occur with two solid phases and one liquid phase, with two solid phases and one gas phase, or with three solid phases. According to the Gibbs phase rule, a three-phase situation in a one-component system has no degrees of freedom (that is, it is invariant). Consequently, a triple point occurs at a unique temperature and pressure, because any change in either variable will result in the disappearance of at least one of the three phases. See also: PHASE EQUILIBRIUM . Triple points are shown in the illustration of part of the phase diagram for water. Point A is the well-known ◦ triple point for Ice I (the ordinary low-pressure solid form) + liquid + water + water vapor at 0.01 C (273.16 K) and a pressure of 0.00603 atm (4.58 mmHg or 611 pascals). In 1954 the thermodynamic temperature scale (the absolute or Kelvin scale) was redefined by setting this triple-point temperature for water equal to exactly 273.16 K. Thus, the kelvin (K), the unit of thermodynamic temperature, is defined to be 1 ∕ 273.16 of the thermodynamic temperature of this triple point. ◦ Point B , at 251.1 K ( − 7.6 F) and 2047 atm (207.4 megapascals) pressure, is the triple point for liquid water + Ice ◦ I + Ice III; and point C , at 238.4 K ( − 31 F) and 2100 atm (212.8 MPa) pressure, is the triple point for Ice I + Ice II + Ice III. -
Superconductivity for Accelerators
Superconductivity for accelerators - why bother? Abolish Ohm's • no power consumption Law(although do need refrigeration power) • high current density ⇒ compact windings, high gradients • ampere turns are cheap, so don’t need iron ⇒ high fields (although often use it for shielding) Consequence • lower power bills s • higher magnetic fields mean reduced bend radius ⇒ smaller rings ⇒ reduced capital cost ⇒ new technical possibilities (eg muon collider) • higher quadrupole gradients ⇒ higher luminosity Martin Wilson Lecture 1 slide 1 JUAS February 2015 Superconducting Magnets: plan of the lectures 1 Introduction to Superconductors 4 Quenching and Protection • critical field, temperature & current • the quench process • superconductors for magnets • resistance growth, current decay, • manufacture of superconducting wires temperature rise • high temperature superconductors HTS • calculating the quench • where to find more • mini tutorial 2 Magnetization, Cables & AC losses • quench protection schemes • superconductors in changing fields, critical state model • filamentary superconductors and magnetization 5 Cryogenics & Practical Matters • coupling between filaments ⇒ magnetization • working fluids, refrigeration • why cables, coupling in cables • cryostat design • mini tutorial • current leads • AC losses in changing fields • accelerator magnet manufacture 3 Magnets, ‘Training’ & Fine Filaments • some superconducting accelerators • coil shapes for solenoids, dipoles & quadrupoles • engineering current density & load lines mini tutorials • -

Thermodynamic Curvature from the Critical Point to the Triple Point
Thermodynamic curvature from the critical point to the triple point George Ruppeiner∗ Division of Natural Sciences New College of Florida 5800 Bay Shore Road Sarasota, Florida 34243-2109 October 30, 2018 Abstract I evaluate the thermodynamic curvature R for fourteen pure fluids along their liquid-vapor coexistence curves, from the critical point to the triple point, using thermodynamic input from the NIST Chem- istry WebBook. In this broad overview, R is evaluated in both the coexisting liquid and vapor phases. R is an invariant whose magni- tude jRj is a measure of the size of mesoscopic organized structures in a fluid, and whose sign specifies whether intermolecular interactions are effectively attractive (R < 0) or repulsive (R > 0). I discuss five principles for R in pure fluids: 1) near the critical point, the attractive part of the interactions forms loose structures of size jRj proportional to the correlation volume ξ3, and sign of R negative, 2) in the vapor phase, there are instances of compact clusters of size jRj formed by the attractive part of the interactions and prevented from collapse by the repulsive part of the interactions, and sign of R positive, 3) in the asymptotic critical point regime, the R's in the coexisting liquid and arXiv:1208.3265v1 [cond-mat.stat-mech] 16 Aug 2012 vapor phases are equal to each other, i.e., commensurate, 4) outside the asymptotic critical point regime incommensurate R's may be as- sociated with metastability, and 5) the compact liquid phase has jRj ∗[email protected] 1 on the order of the volume of a molecule, with sign of R negative for a liquidlike state held together by attractive interactions and sign of R positive for a solidlike state held up by repulsive interactions. -

Triple Point of Water
Demonstration 4 Latent Heat of Vaporization The Triple Point of Water gas phase liquid + gas liquid phase solid + liquid solid phase Temperature Time When a liquid is boiling, molecules are constantly leaving the surface and in the process changing from a liquid state to a gas state. As each molecule leaves the surface it will take some thermal energy with it. The amount of thermal energy will be determined by the heat required to reach the boiling point of the liquid plus the latent heat of vaporization defined as “the quantity of heat required to change unit mass of liquid into vapor at the boiling point”. Normally, the thermal energy necessary to reach and sustain boiling is supplied to the liquid from an external source. If instead, the liquid is made to boil by reducing the pressure over the surface with no external heat provided, the process is reversed. The molecules leaving the liquid surface and entering the gas state still require their latent heat as before; but, since no heat is being supplied, the thermal energy is obtained from the molecules that remain in the liquid state. This will result in a reduction in the kinetic energy of the remaining molecules of liquid. The loss of kinetic energy will cause a drop in the temperature of the remaining liquid. If enough cooling occurs, the liquid will undergo yet another phase change to a solid. When all three phases are present and in equilibrium, the temperature and pressure condi- tions at which this occurs is called the “triple point”. Once the triple-point is reached, additional pumping will remove the gas phase and the solid phase (ice in the case of water) will remain. -

Pressure-Induced Superconductivity in the Three-Component Fermion
Pressure-induced Superconductivity in the Three-component Fermion Topological Semimetal Molybdenum Phosphide Zhenhua Chi1, Xuliang Chen2, Chao An2,3, Liuxiang Yang4, Jinggeng Zhao5,6, Zili Feng7,8, Yonghui Zhou2, Ying Zhou2,3, Chuanchuan Gu2, Bowen Zhang2,3, Yifang Yua n 2,9, Curtis Kenney-Benson10, Wenge Yang 11, Gang Wu12, Xiangang Wan13,14, Youguo Shi7,¦, Xiaoping Yang2,$, Zhaorong Yang2,14,§ 1Key Laboratory of Materials Physics, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China 2Anhui Province Key Laboratory of Condensed Matter Physics at Extreme Conditions, High Magnetic Field Laboratory, Chinese Academy of Sciences, Hefei 230031, China 3University of Science and Technology of China, Hefei 230026, China 4Center for High Pressure Science and Technology Advanced Research (HPSTAR), Beijing 100094, China 5Department of Physics, Harbin Institute of Technology, Harbin 150080, China 6Natural Science Research Center, Academy of Fundamental and Interdisciplinary Sciences, Harbin Institute of Technology, Harbin 150080, China 7Beijing National Laboratory for Condensed Matter Physics and Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China 8School of Physical Sciences, University of Chinese Academy of Sciences, Beijing 100190, China 9Department of Physics and Engineering, Zhengzhou University, Zhengzhou 450052, China 10HPCAT, Geophysical Laboratory, Carnegie Institution of Washington, Argonne, Illinois 60439, USA 11Center for High Pressure Science and Technology Advanced Research (HPSTAR), -

Physical Chemistry Icarnot Cycle
Lecture 17 Chapter 9, sections 1-3 Announce: • Remember to complete and return Dr. Krueger’s mid-term evaluations forms • Chem seminar on Friday Outline: Phase Transitions ∂G = −S ∂ T p Phase Diagrams (H2O and CO2) ∂G = V ∂ p T Chemical Potential ∂G µ = ∂ n p,T Review: Some protein and nucleic acid binding events are driven by enthalpy and some by entropy. The balance between the two is governed by the solvent interactions: favorable solvent interactions Æ enthalpy controlled poor solvent interactions Æ entropy controlled The body makes nonspontaneous reactions happen by coupling them to exergonic reactions like ATP Æ ADP Phase Diagrams Phase Diagrams Summarize Solid-Liquid-Gas Behavior of a Substance Let’s start by defining a phase: form of matter that is uniform throughout in chemical composition and physical state. phase transition: the spontaneous conversion of one phase to another that occurs at a well-defined temperature – the transition temperature said another way, the transition temperature is the temperature at which the Gibbs free energies for both phases are? equal (remember that the system is seeking the minimum Gibbs free energy). 1 We can explain transition temperatures by understanding how the Gibbs energy changes with temperature: Recall that G is a function of p and T, so then the total derivative is? ∂G ∂G dG = dp + dT (from definition of total derivative for dG) ∂p ∂T T p But, we also know from our fundamental equation for G?: dG = Vdp - SdT As we have done three times before, you guys can quickly fill in? ∂G ∂G = Vand =−S (equating coefficients in expressions for dG) ∂p ∂T T p Therefore, if we plot G vs. -
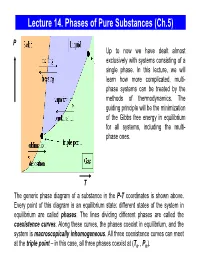
Lecture 14. Equilibrium Between Phases
Lecture 14. Phases of Pure Substances (Ch.5) P Up to now we have dealt almost exclusively with systems consisting of a single phase. In this lecture, we will learn how more complicated, multi- phase systems can be treated by the methods of thermodynamics. The guiding principle will be the minimization of the Gibbs free energy in equilibrium for all systems, including the multi- phase ones. T The generic phase diagram of a substance in the P-T coordinates is shown above. Every point of this diagram is an equilibrium state; different states of the system in equilibrium are called phases. The lines dividing different phases are called the coexistence curves. Along these curves, the phases coexist in equilibrium, and the system is macroscopically inhomogeneous. All three coexistence curves can meet at the triple point – in this case, all three phases coexist at (Ttr , Ptr). The Coexistence Curves Along the coexistence curves, two different phases 1 and 2 coexist in equilibrium (e.g., ice and water coexist at T = 00C and P = 1bar). The system undergoes phase separation each time we cross the equilibrium curve (the system is spatially inhomogeneous along the equilibrium curves). Any system in contact with the thermal bath is governed by the minimum free energy principle. The shape of coexistence curves on the P-T phase diagram is determined by the condition: GPTGPT,,= and, since 1()2 ( ) GN= μ PTPTμ1( ,,) = μ2 ( ) - otherwise, the system would be able to decrease its Gibbs free energy by transforming the phase with a higher μ into the phase with lower μ. -

Make a Triple Point Apparatus Student Laboratory SCIENTIFIC
Make a Triple Point Apparatus Student Laboratory SCIENTIFIC Introduction View carbon dioxide in the solid, liquid, and gas forms simultaneously using a simple triple point apparatus made by you right in the laboratory! Concepts • Sublimation • Phase diagrams • Triple point Background We have all observed what occurs when dry ice (solid state of carbon dioxide, 72.8 CO2) is removed from a freezer. As the temperature on the surface of the dry Solid Liquid ice increases above –78 °C, the solid changes directly to a vapor bypassing the liquid phase. This spontaneous change from a solid to a gas is called sublimation. Sublimation of CO2 occurs when the temperature is above –78 °C at a pressure of 1 atm, standard atmospheric pressure. Standard 67 pressure on Earth is 1 atm. Liquid CO2 is not stable under Earth’s standard pressure, and therefore does not exist under normal pressure conditions. Refer 5.1 to the phase diagram, Figure 1. Pressure (atm) A phase diagram has temperature as the independent (x) and pressure as the 1 Gas dependent (y) axis. According to the phase diagram that the liquid phase of CO 2 –78 –56 25 31 is stable only under a pressure of 5.2 atm or higher, at a temperature above Temperature ( C) –57 °C. The junction where the gas, liquid, and solid phases all exist in ϒ equilibrium is called the triple point. When a substance is at the triple point, all Figure 1. Phase diagram of carbon three phases are present simultaneously. dioxide. The terms melting point (solid to liquid) and boiling point (liquid to gas) are often used when discussing phase changes.