The Crab Spider–Pitcher Plant Relationship Is a Nutritional Mutualism That Is Dependent on Prey- Resource Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OF RAJASTHAN with SPECIAL REFERENCE to RANTHAMBORE NATIONAL PARK, RAJASTHAN, INDIA Sumana Saha, D
© Indian Society of Arachnology ISSN 2278-1587(Online) SPIDER FAUNA (ARANEAE: ARACHNIDA) OF RAJASTHAN WITH SPECIAL REFERENCE TO RANTHAMBORE NATIONAL PARK, RAJASTHAN, INDIA Sumana Saha, D. C. Dhali* and D. Raychaudhuri* Department of Zoology, Darjeeling Govt. College, Darjeeling, Govt. of West Bengal, India. email: [email protected] *Entomology Laboratory, Department of Zoology, University of Calcutta, 35, Ballygunge Circular Road, Kolkata- 700019, India. email: [email protected], [email protected] corresponding author: [email protected] ABSTRACT 12 species of 10 genera belonging to 9 families were recorded from Ranthambore National Park, during 2008. Thomisus italongus Barrion and Litsinger and Cyrtophora exanthematica (Doleschall), are recognized as new from the country and accordingly described and illustrated. Key words: Spiders, New records, Ranthambore National Park, Rajasthan, India. INTRODUCTION Protected areas are essential for biodiversity conservation. They act as benchmarks to understand human interaction with the natural world. Of the two mega diverse Indian states, Rajasthan with an area of about 342,239 sq. km. is the largest state of India. The state is symbolized by its arid and semi-arid tracts. Ecologically suitable forest areas of the state over time have been declared as National Parks, Wild Life Sanctuaries and Reserves. Wildlife wing of the State Forest Department (SFD) maintain these protected areas in a scientific manner, conducive for conservation of wild life in the area. Temporal population of any life form actively select a suitable site for sustainance. Its fitness is directly influenced by the ability to find a suitable habitat based on innate preference for food, shelter and absence of enemies. -
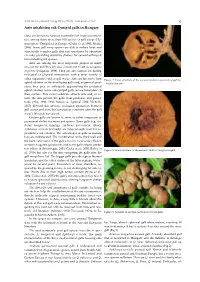
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

Crab Spiders Impact Floral-Signal Evolution Indirectly Through Removal
ARTICLE DOI: 10.1038/s41467-018-03792-x OPEN Crab spiders impact floral-signal evolution indirectly through removal of florivores Anina C. Knauer1, Moe Bakhtiari1,2 & Florian P. Schiestl1 The puzzling diversity of flowers is primarily shaped by selection and evolutionary change caused by the plant’s interaction with animals. The contribution of individual animal species to net selection, however, may vary depending on the network of interacting organisms. Here 1234567890():,; we document that in the buckler mustard, Biscutella laevigata, the crab spider Thomisus onustus reduces bee visits to flowers but also benefits plants by feeding on florivores. Uninfested plants experience a trade-off between pollinator and spider attraction as both bees and crab spiders are attracted by the floral volatile β-ocimene. This trade-off is reduced by the induced emission of β-ocimene after florivore infestation, which is stronger in plant populations where crab spiders are present than where they are absent, suggesting that plants are locally adapted to the presence of crab spiders. Our study demonstrates the context-dependence of selection and shows how crab spiders impact on floral evolution. 1 Department of Systematic and Evolutionary Botany, University of Zurich, Zollikerstrasse 107, 8008 Zurich, Switzerland. 2Present address: Institute of Biology, University of Neuchatel, Rue Emile-Argand 11, 2000 Neuchatel, Switzerland. Correspondence and requests for materials should be addressedto F.P.S. (email: fl[email protected]) NATURE COMMUNICATIONS | (2018) 9:1367 | DOI: 10.1038/s41467-018-03792-x | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-03792-x lant–animal interactions are a major driver of plant Crab spiders camouflage themselves on flowers to hunt flower- evolution, including both local adaptation and species visiting insects such as pollinators (Fig. -

RE-DESCRIPTION of the CRAB SPIDER, Thomisus Citrinellus Simon, 1875 (ARANEAE, THOMISIDAE) from EGYPT Gihan M
© Indian Society of Arachnology ISSN 2278 - 1587 RE-DESCRIPTION OF THE CRAB SPIDER, Thomisus citrinellus Simon, 1875 (ARANEAE, THOMISIDAE) FROM EGYPT Gihan M. E. Sallam Plant Protection Research Institute, Agricultural Research Center, Dokki, Giza, Egypt E-mail:[email protected] ABSTRACT Two males and one female of Thomisus citrinellus Simon, 1875 (Araneae: Thomisidae) were re-described.The specimens were collected from Egypt. The earlier description was done by Levy in 1985 based on specimens collected from Israel. INTRODUCTION Thomisidae is a large family distributed worldwide, comprising 174 genera and 2151 species (Platnick, 2012) all over the world. Of these, 10 genera and 25 species are known from Egypt (El-Hennawy, 2006). Genus Thomisus Walckenaer, 1805 includes 22 species from Africa (Platnick, 2012) and they are as follows: albohirtus Simon, 1884; australis Comellini, 1957; bidentatus Kulczyn’ski, 1901; blandus Karsch, 1880; candidus Blackwall, 1866; citrinellus Simon, 1875; congoensis Comellini, 1957; dalmasi Lessert, 1919; daradioides Simon, 1890; daradioides nigroannulatus Caporiacco, 1947; granulatus Karsch, 1880; kalaharinus Lawrence, 1936; kiwuensis Strand, 1913; litoris Strand, 1913; machadoi Comellini, 1959f obscuratus Caporiacco, 1947; onustus meridionalis Strand, 1907; schultzei Simon, 1910; scrupeus (Simon, 1886); stenningi Pocock, 1900; tripunctatus Lucas, 1858 and zuluanus Lawrence, 1942. The members of this family are commonly called the crab spiders as the first two legs are strong and laterigrade. They are usually found on trees, shrubs, and grasses, especially on flowers, as well as in leaf litter and under stones on the ground (Foelix, 1996). Genus Thomisus has a truncated cephalothorax in front, with the upper fore-corners strongly and conically protuberant and divergent, bearing the lateral eyes. -

The Leafhoppers of Minnesota
Technical Bulletin 155 June 1942 The Leafhoppers of Minnesota Homoptera: Cicadellidae JOHN T. MEDLER Division of Entomology and Economic Zoology University of Minnesota Agricultural Experiment Station The Leafhoppers of Minnesota Homoptera: Cicadellidae JOHN T. MEDLER Division of Entomology and Economic Zoology University of Minnesota Agricultural Experiment Station Accepted for publication June 19, 1942 CONTENTS Page Introduction 3 Acknowledgments 3 Sources of material 4 Systematic treatment 4 Eurymelinae 6 Macropsinae 12 Agalliinae 22 Bythoscopinae 25 Penthimiinae 26 Gyponinae 26 Ledrinae 31 Amblycephalinae 31 Evacanthinae 37 Aphrodinae 38 Dorydiinae 40 Jassinae 43 Athysaninae 43 Balcluthinae 120 Cicadellinae 122 Literature cited 163 Plates 171 Index of plant names 190 Index of leafhopper names 190 2M-6-42 The Leafhoppers of Minnesota John T. Medler INTRODUCTION HIS bulletin attempts to present as accurate and complete a T guide to the leafhoppers of Minnesota as possible within the limits of the material available for study. It is realized that cer- tain groups could not be treated completely because of the lack of available material. Nevertheless, it is hoped that in its present form this treatise will serve as a convenient and useful manual for the systematic and economic worker concerned with the forms of the upper Mississippi Valley. In all cases a reference to the original description of the species and genus is given. Keys are included for the separation of species, genera, and supergeneric groups. In addition to the keys a brief diagnostic description of the important characters of each species is given. Extended descriptions or long lists of references have been omitted since citations to this literature are available from other sources if ac- tually needed (Van Duzee, 1917). -

A Summary List of Fossil Spiders
A summary list of fossil spiders compiled by Jason A. Dunlop (Berlin), David Penney (Manchester) & Denise Jekel (Berlin) Suggested citation: Dunlop, J. A., Penney, D. & Jekel, D. 2010. A summary list of fossil spiders. In Platnick, N. I. (ed.) The world spider catalog, version 10.5. American Museum of Natural History, online at http://research.amnh.org/entomology/spiders/catalog/index.html Last udated: 10.12.2009 INTRODUCTION Fossil spiders have not been fully cataloged since Bonnet’s Bibliographia Araneorum and are not included in the current Catalog. Since Bonnet’s time there has been considerable progress in our understanding of the spider fossil record and numerous new taxa have been described. As part of a larger project to catalog the diversity of fossil arachnids and their relatives, our aim here is to offer a summary list of the known fossil spiders in their current systematic position; as a first step towards the eventual goal of combining fossil and Recent data within a single arachnological resource. To integrate our data as smoothly as possible with standards used for living spiders, our list follows the names and sequence of families adopted in the Catalog. For this reason some of the family groupings proposed in Wunderlich’s (2004, 2008) monographs of amber and copal spiders are not reflected here, and we encourage the reader to consult these studies for details and alternative opinions. Extinct families have been inserted in the position which we hope best reflects their probable affinities. Genus and species names were compiled from established lists and cross-referenced against the primary literature. -

The Leafhoppers, Or Cicadellidae, of Illinois (Eurymelinae-Balcluthinae)
BULLETIN of the ILLINOIS NATURAL HISTORY SURVEY HARLOW B. MILLS, Chief The Leafhoppers, or Cicadellidae, of Illinois (Eurymelinae-Balcluthinae) D. M. DELONG PriDted by Authority of the STATE OF ILLINOIS DWIGHT H. GREEN, Govtrnor DEPARTMENT OF REGISTRATION AND EDUCATION FRANK G. THOMPSON, Dirtctor STATE t) F I 1. I, I N O I S DwiGiiT H. CiREES', Governor PEPARTMENT OF REGISTRATION ANi:) EDUCATION Frank G. Thompson, Director \^ ^- \' N A T U R A L HISTORY S U R E I ) I 1 S I O N Hari.o\\ B. Mii.i.s, (-liicf \ olumc 24 BULI^K TIN Article 2 The Leafhoppers, or Cicadellidae, of Illinois (Eurymelinae— Balcluthinae) ]). M. 1)1 f,c)Nc; Priulid hy Jul/iority of the Stall- of Illinois URBANA, ILLINOIS June 194S STATE OF ILLINOIS DwiGHT H. Green, Governor DEPARTMENT OF REGISTRATION AND EDUCATION Frank G. Thompson, Director BOARD OF NATURAL RESOURCES AND CONSERVATION Frank G. Thompson, Chairman A. E. Emerson, Ph.D., Rio/oxv George D. Stoddard, Ph.D., Litt.D., L.H.D., L. H Tiffany, Ph.D., Forestry LL.D., President of the Ihiivcrsily nj Illinois l' R. Howson, B.S.C.E., C.E., Walter H. Newhoi'isk, Ph.D., Geology Engineering Roger Adams, Ph.D., D.Sc, Chemistry NATURAL HISTORY SURVEY DIVISION Urbana, Illinois Scientific and Technical Staff H.^Ri.ow B. Mills, Ph.D., Chief Bessie B. Henderson, M.S., Assistant to the Chief Section of Economic Entomology Section of Forestry Entomologist George C. Decker, Ph.D., WiLLET N. Wandell, M.F., Forester and and Head Head M.S., Entomologist J. -

A Five-Year Research Program Is Proposed to Expand the Theory of Community Assembly from Its Current Base of Correlative Inferen
PROJECT SUMMARY A five-year research program is proposed to expand the theory of community assembly from its current base of correlative inferences to one grounded in process-based conclusions derived from controlled field and laboratory experiments. Northern pitcher plants, Sarracenia purpurea, and their community of inquiline arthropods and rotifers, will be used as the model system for the proposed experiments. There are three goals to the proposed research. (1) Inquiline assemblages that colonize pitcher plants will be developed as a model system for understanding community assembly and persistence. (2) Field and laboratory experiments will be used to elucidate causes of inquiline community colonization, assembly, and persistence, and the consequences of inquiline community dynamics for plant leaf allocation patterns, growth, and reproduction, as well as within-plant nutrient cycling. Reciprocal interactions of plant dynamics on inquiline community structure will also be investigated experimentally. (3) Matrix models will be developed to describe reciprocal interactions between inquiline community assembly and persistence, and inquilines’ living host habitats. As an integrated whole, the proposed experiments and models will provide a complete picture of linkages between pitcher-plant inquiline communities and their host plants, at individual leaf and whole-plant scales. This focus on measures of plant performance will fill an apparent lacuna in prior studies of pitcher plant microecosystems, which, with few exceptions, have focused almost exclusively on inquiline population dynamics and interspecific interactions. Plant demography of S. purpurea will be described and modeled for the first time. Complementary, multi-year field and greenhouse experiments will reveal effects of soil and pitcher nutrient composition on leaf allocation, plant growth, and reproduction. -

Folk Taxonomy, Nomenclature, Medicinal and Other Uses, Folklore, and Nature Conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3
Ulicsni et al. Journal of Ethnobiology and Ethnomedicine (2016) 12:47 DOI 10.1186/s13002-016-0118-7 RESEARCH Open Access Folk knowledge of invertebrates in Central Europe - folk taxonomy, nomenclature, medicinal and other uses, folklore, and nature conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3 Abstract Background: There is scarce information about European folk knowledge of wild invertebrate fauna. We have documented such folk knowledge in three regions, in Romania, Slovakia and Croatia. We provide a list of folk taxa, and discuss folk biological classification and nomenclature, salient features, uses, related proverbs and sayings, and conservation. Methods: We collected data among Hungarian-speaking people practising small-scale, traditional agriculture. We studied “all” invertebrate species (species groups) potentially occurring in the vicinity of the settlements. We used photos, held semi-structured interviews, and conducted picture sorting. Results: We documented 208 invertebrate folk taxa. Many species were known which have, to our knowledge, no economic significance. 36 % of the species were known to at least half of the informants. Knowledge reliability was high, although informants were sometimes prone to exaggeration. 93 % of folk taxa had their own individual names, and 90 % of the taxa were embedded in the folk taxonomy. Twenty four species were of direct use to humans (4 medicinal, 5 consumed, 11 as bait, 2 as playthings). Completely new was the discovery that the honey stomachs of black-coloured carpenter bees (Xylocopa violacea, X. valga)were consumed. 30 taxa were associated with a proverb or used for weather forecasting, or predicting harvests. Conscious ideas about conserving invertebrates only occurred with a few taxa, but informants would generally refrain from harming firebugs (Pyrrhocoris apterus), field crickets (Gryllus campestris) and most butterflies. -

Chemical Deception Among Ant Social Parasites
Current Zoology 60 (1): 62–75, 2014 Chemical deception among ant social parasites Rhian M. GUILLEM1, Falko DRIJFHOUT2, Stephen J. MARTIN3* 1 Department of Animal and Plant Sciences, University of Sheffield, S10 2TN, UK 2 Chemical Ecology Group, School of Physical and Geographical Sciences, Lennard-Jones Laboratory, Keele University, ST5 5BG, UK 3 School of Environment and Life Sciences, University of Salford, Manchester M5 4WT, UK Abstract Deception is widespread throughout the animal kingdom and various deceptive strategies are exemplified by social parasites. These are species of ants, bees and wasps that have evolved to invade, survive and reproduce within a host colony of another social species. This is achieved principally by chemical deception that tricks the host workers into treating the invading parasite as their own kin. Achieving levels of acceptance into typically hostile host colonies requires an amazing level of decep- tion as social insects have evolved complex species- and colony-specific recognition systems. This allows the detection of for- eigners, both hetero- and con-specific. Therefore, social parasitic ants not only have to overcome the unique species recognition profiles that each ant species produces, but also the subtle variations in theses profiles which generate the colony-specific profiles. We present data on the level of chemical similarity between social parasites and their hosts in four different systems and then discuss these data in the wider context with previous studies, especially in respect to using multivariate statistical methods when looking for differences in these systems [Current Zoology 60 (1): 6275, 2014]. Keywords Mimicry, Social parasites, Cuticular hydrocarbons, Multivariate statistics Deception by mimicking the pattern of another spe- 1968). -

The Adaptive Significance of Inquiline Parasite Workers
Received 5December 2002 Accepted 29January 2003 Publishedonline 30April 2003 Theadaptive significance of inquilineparasite workers Seirian Sumner * ,David R.Nash and Jacobus J.Boomsma Departmentof Population Ecology, Zoological Institute, University ofCopenhagen, Universitetsparken 15, DK-2100Copenhagen, Denmark Social parasites exploit thesocially managed resourcesof their host’s society.Inquiline social parasites are dependenton their hostthroughout their life cycle,and so many ofthe traits inherited from their free-living ancestorare removedby natural selection.One trait that is commonly lostis theworker caste, thefunctions of which are adequately fulfilled by hostworkers. The fewinquiline parasites that have retaineda worker casteare thought tobe at atransitional stage in theevolution of social parasitism, and their worker castesare consideredvestigial andnon-adaptive. However, this idea has notbeen tested. Furthermore, whetherinquiline workershave anadaptive role outsidethe usual worker repertoire of foraging, broodcare andcolony maintenance has notbeen examined. In this paper, wepresentdata that suggestthat workersof the inquiline ant Acromyrmexinsinuator play avital role in ensuringthe parasite’ s fitness.We show that thepresence of these parasite workershas apositive effecton the production of parasite sexualsand a negative effecton the production of host sexuals. This suggeststhat inquiline workersplay avital role in suppressinghost queen reproduction, thus promoting therearing ofparasite sexuals.To ourknowledge, these are thefirst -

Download PDF File
Myrmecological News 20 7-14 Vienna, September 2014 Imperfect chemical mimicry explains the imperfect social integration of the inquiline ant Ectatomma parasiticum (Hymenoptera: Formicidae: Ectatomminae) Fabrice SAVARIT & Renée FÉNÉRON Abstract Inquilinism is an extreme form of social parasitism where the parasite permanently depends on its host. This parasitism is widespread among Formicinae and Myrmicinae ants but rare in other subfamilies, like Ectatomminae where Ectatomma parasiticum FEITOSA & FRESNEAU, 2008 is the sole inquiline described so far. This species is genetically and morpholo- gically very similar to its host, E. tuberculatum (OLIVIER, 1792), and uses its worker force to produce exclusively sexuals. During their life cycle, E. parasiticum queens enter into established host colonies and cohabit with the resident queens over an extended period of time. However, previous experiments in the laboratory have shown that some parasites are attacked by host workers, suggesting that their social integration into host colonies is incomplete or unstable. We thus investigate how the chemical cues of the parasites relate to the host's recognition system. For this, we compare the cuticu- lar hydrocarbon profiles of parasites, host queens and host workers using solid-phase microextraction. Although over- lapping, the chemical profiles of both species are distinct. Parasites have no specific compounds but a reduced total amount of cuticular hydrocarbons compared with hosts. We suggest that E. parasiticum uses an imperfect chemical mimicry stra- tegy as it is well-discriminated by its host species. Key words: Chemical strategy, cuticular hydrocarbons, inquiline ants, Ectatomma parasiticum, Ectatomma tuberculatum. Myrmecol. News 20: 7-14 (online 17 February 2014) ISSN 1994-4136 (print), ISSN 1997-3500 (online) Received 14 June 2013; revision received 12 November 2013; accepted 12 November 2013 Subject Editor: Falko P.