A Cadaveric Study of Coronary Artery Variations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Development and Teratology of Cardiovascular and Lymphatic Systems
Development and teratology of cardiovascular and lymphatic systems Repetition: Muscle tissue Beginning of the cardiovascular system development – the 3rd week: Hemangiogenesis (day 15 – 16) – blood islets (insulae sanguinae) in extraembryonic mesoderm and splanchnic mesenchyme of embryo Clusters of mesenchyme cells (angiogenic cells) differentiate into: - angioblasts endothelium (at the periphery of blood islets) - hemoblasts primitive erythrocytes (in the center of blood islets) Clusters of angiogenic cells form a "horseshoe-shaped" space between somatic and splanchnic layer of mesoderm = pericardial cavity. Two endothelial tubes arrise in splanchnic mesoderm. The ventral portion of these tubes forms the cardiogenic area with two heart tubes, while the lateral portions form the dorsal aortae. Germ disc: prosencephalon mesencephalon eye rhombencephalon heart lateral mesoderm somites small blood vessels blood islands 8,9 Spine primitive streak Initially, the cardiogenic area is located anterior to the prechordal plate and the neural plate. The growth of the central nervous system pulls the cardiogenic area and prechordal plate (buccopharyngeal membrane ventrally and caudally ( ). Cardiogenic region just cranial to the prechordal plate. The canalization of cardiogenic clusters in the splanchnic mesoderm results in the formation of the paired heart tubes. Folding of embryo and primitive gut separation from yolk sac. Fusion of the heart tubes a single heart tube is, temporarily attached to the dorsal side of the pericardial cavity by the -

Model Keys Unit 1
Model Keys This section will supply you with the keys to several of the models found in the anatomy lab and the learning center. This does not include all the models. After the model keys in this section you will find keys to most of the Nystrom charts. In the anatomy lab there is a reference shelve that contains binders with the keys to most models, torsos and the Nystrom charts. Keys to some of the models are actually attached to the model. Chart keys can also be found either right on the chart or posted next to the chart. Unit 1 Numbered Skull (with colored numbers): g. internal occipital crest a. third molar (inside) b. incisive canal h. clivus (inside) c. infraorbital foramen i. foramen magnum d. infraorbital groove j. jugular foramen e. pterygopalatine fossa k. hypoglossal canal 6. Zygomatic bone l. cerebella fossa (inside) a. zygomaticofacial foramen m. vermain fossa (inside) 7. Sphenoid bone 4. Temporal bone a. medial pterygoid plate a. styloid process b. lateral pterygoid plate b. tympanic part c. lesser wing of sphenoid c. mastoid process (inside) d. mastoid notch d. greater wing of sphenoid 1. Frontal bone e. zygomatic process (inside) a. zygomatic process f. articular tubercle e. chiasmatic groove b. frontal crest (inside) g. stylomastoid foramen (inside) c. orbital part (inside) h. mastoid foramen f. anterior clinoid process d. supraorbital foramen i. external acoustic meatus (inside) e. anterior ethmoidal j. internal acoustic meatus g. posterior clinoid process foramen (inside) (inside) f. posterior ethmoidal k. carotid canal h. hypophyseal fossa foramen l. mandibular fossa (inside) g. -

Pericardium & HEART
Slides of Anatomy Please note : These slides were edited by our colleague Sara Rahhal to fit the slides of spring 2019. Pericardium & HEART Dr.Maher AL-Hadidi School of Medicine University of Jordan Spring 2019 Spring2019 Dr,Maher AL-Hadidi , University ofJordan 1 [Type here] Pericardium A double-walled fibroserous conical-shaped sac, inside middle mediastinum. Enclose the heart and roots of its large vessels. PericardiumVagusnerves Pericardium Pericardium SVC PericardiophrenicA. PericardiophrenicV. MusculophRt.renic Pericardiophrenic vessels branches Diaphragm Base [Type here] Diaphragm 2 Pericardium – Sagittal section Parts: 1 1. Fibrous pericardium. (outer) 2. Serous pericardium. 2 Fibrous pericardium (Inner) Conical-shaped fibrous sac. Base: Attached to central tendon of diaphragm. Layers . Apex: Attached to roots of large ofheart vessels. Wall Prevent overextension of the Base heart. (potential) [Typehere] Serous pericardium Complete serous sac invaginated by 1 the heart. Like pleura by the lung. (outer) 2 layers: 2 Parietal layer lines fibrous pericardium. (Inner) Visceral layer covers the heart as (Epicardium). Layers ofheart Pericardial cavity: Wall A potential space between 2 serous Base layers. (potential) [Typehere] Contents of pericardium: Superior vena cava Contents: Arch of aorta 1. Heart and all arteries, Ascending Aorta Transverse sinus Pulmonary veins and nerves. (separatesarteries Artery fromveins) 2 3 2. All Ascending aorta. 4 8 Left pulmonary 3. All Pulmonary Artery. 7 Right pulmonary vs. 9 6 4. Last 2cm of SVC. 1 5. Last 2cm of IVC. Serous pericardium + Oblique sinus 5 6. First part of the 4 diaphragm Fibrous pericardium pulmonary veins. Inferior vena cava 7. Transverse sinus. Thoracic aorta 8. Oblique sinus. Dr. Maher Hadidi, University of Jordan Heart Pyramidal-shaped muscular organ. -

Thorax, Abdomen, Pelvis
GERARD GORNIAK & WILLIAM CONRAD HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS Download free eBooks at bookboon.com 2 Human Anatomy Synopsis: Thorax, Abdomen, Pelvis 1st edition © 2018 Gerard Gorniak & William Conrad & bookboon.com ISBN 978-87-403-2213-2 Peer review by Dr. Edward Kane, University of St. Augustine, USA Download free eBooks at bookboon.com 3 HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS CONTENTS CONTENTS Preface 7 1 Thoracic Cage 8 1.1 Boundaries 8 1.2 Osteology 8 1.3 Muscles of the Thorax 16 1.4 Intercostal Nerves (Fig. 1-13) 30 1.5 Intercostal Arteries and Veins (Figs. 1-13, 1-16, 1-17) 31 2 The Lungs 35 2.1 The Pleura (Fig. 2-2) 36 2.2 Lobes of the Lung (Figs 2-3, 2-4) 38 2.3 Pulmonary Vessels (Figs. 2-9, 2-10) 45 Free eBook on Learning & Development By the Chief Learning Officer of McKinsey Download Now Download free eBooks at bookboon.com Click on the ad to read more 4 HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS CONTENTS 3 Heart 49 3.1 Mediastinum (Fig. 3-1) 49 3.2 Pericardium (Fig. 3-2) 51 3.3 Heart Overview (Fig. 3-3) 51 3.4 Structure of Arteries and Veins (Fig. 15-14) 67 4 Superior And Posterior Mediastina 72 4.1 Superior Mediastinum 72 4.2 Posterior Mediastinum 76 5 Abdominal Wall 84 5.1 Boundaries 84 5.2 Abdominal Planes (Table 4.1 and Fig. 4-1) 84 5.3 Anterior and Lateral Abdominal Walls 87 5.4 Inguinal Region (Figs. -

The Original Heart of America ™ #140, A40 1
THE ORIGINAL HEART OF AMERICA ™ #140, A40 1. Right atrium 48. Apex of heart 2. Right auricle 49. Circumflex branch of left coronary artery 3. Coronary sulcus 50. Anterior interventricular branch of left 4. Right ventricle coronary artery 5. Anterior interventricular sulcus 51. Left auricle 6. Left ventricle 52. Pectinate muscle 7. Left atrium 53. Crista terminalis 8. Pulmonary veins 54. Posterior left ventricular branch 9. Pulmonary trunk 55. Fossa ovalis (foramen ovale**) a. Right branch of pulmonary artery 56. Limbus of fossa ovalis b. Left branch of pulmonary artery 57. Valve of coronary sinus 10. Ligamentum arteriosum 58. Opening of coronary sinus (ductus arteriosus*) 59. Azygous vein 11. Ascending aorta 60. Middle cardiac vein 12. Aortic arch 61. Posterior interventricular branch 13. Brachiocephalic trunk 62. Trabeculae carneae 14. Left common carotid artery 63. Marginal branches on right coronary artery 15. Left subclavian artery 16. Superior vena cava 17. Right brachiocephalic vein 18. Left brachiocephalic vein * The ductus arteriosus normally closes at birth and 19. Descending aorta thereafter is referred to as the ligamentum arteriosum. 20. Esophagus 21. Trachea 22. Annular ligament ** The foramen ovale normally closes at birth and 23. Tracheal cartilages thereafter is referred to as the fossa ovalis. 24. Left bronchus 25. Right bronchus 26. Inferior vena cava 27. Coronary sinus 28. Great cardiac vein 29. Left coronary artery 30. Fatty tissue 31. Right coronary artery 32. Sinoatrial node 33. Orifice of inferior vena cava 34. Valve of inferior vena cava 35. Tricuspid valve 36. Atrioventricular node 37. Pulmonary valve (Semilunar valve of pulmonary artery) 38. Right branch of Bundle of His 39. -

Development of Heart Notes
Formation of heart tube: 3rd week Heart beat: 22nd –23rd day (beginning of fourth week) USG detection of heart beat: 7th week Foetal ECG: 11th week Endocardium from original heart tube Myocardium from surrounding mesoderm & epicardium (myoepicardial mantle) (visceral pericardium) Lining of pericardium epithelium of pericardial cavity Transverse sinus formed by disappearance of dorsal mesocardium (Present between arterial and venous ends of the heart tube) FATE Of SINUS VENOSUS Left horn of sinus venosus, along with medial part of common cardinal vein forms coronary sinus Lateral part of common cardinal vein forms oblique vein of left atrium Left venous valve merges with septum secundum. Right venous valve is divided in three parts by appearance of two transverse muscular bands, called limbic bands. i) The part above superior limbic band forms crista terminalis ii) The part between the two bands forms valve of inferior vena cava iii) The part below the inferior limbic band forms valve of coronary sinus INTERATRIAL SEPTUM i) Upper, thicker part is formed by septum secundum ii) Lower, thin part (floor of fossa ovalis) is formed by septum primum iii) Sharp margin of fossa ovalis is formed by lower, curved margin of septum secundum DEVELOPMENT OF RIGHT ATRIUM It develops from 1. Right half of primitive atrial chamber (rough part); 2. Absorption of right horn of sinus venosus (smooth part) and 3. Right atrioventricular canal. DEVELOPMENT OF LEFT ATRIUM It develops from 1. Left half of primitive atrial chamber (rough part – confined to the auricle); 2. Absorption of pulmonary veins (smooth part) and 3. Left atrioventricular canal. -

Morphological Study of Coronary Sinus and Coronary Sinus Ostium in Human Cadaveric Hearts
IF : 4.547 | IC Value 80.26 VolumVolumee : 3-6, | Iss Issueue : 11-4, | AprilNovemb - 2017er 2014 • ISSN • ISSN No N2277o 2277 - 8160 - 8179 Original Research Paper Anatomy Morphological study of coronary sinus and coronary Sinus ostium in human cadaveric hearts ASSISTANT PROFESSOR, DEPARTMENT OF ANATOMY, GOVT.MEDICAL COLLEGE, Dr. N.F.Gathe AKOLA (MAHARASHTRA), INDIA. ASSISTANT PROFESSOR, DEPARTMENT OF ANATOMY, GOVT MEDICAL COLLEGE, Dr. M. S. Supare AKOLA (MAHARASHTRA), INDIA. PROFESSOR & HEAD OF DEPT., DEPARTMENT OF ANATOMY, GOVT.MEDICAL Dr. S. V. Pandit COLLEGE, AKOLA (MAHARASHTRA), INDIA. PROF. & HEAD OF DEPT., DEPARTMENT OF PHYSIOLOGY, GOVT.MEDICAL COLLEGE, Dr. G. G. Aatram AKOLA (MAHARASHTRA), INDIA. ABSTRACT Background: The coronary sinus collects majority of venous blood from the heart & return it to the right atrium of the heart. Few studies were reported on coronary venous system till now. The present study is planned to study the formation, tributaries, length & shape of coronary sinus, and shape, length and breadth of coronary sinus ostium (Thebesian valve). Materials and Methods: Thirty eight formalin xed cadaveric hearts were used for the study. The formation, tributaries, length and shape of coronary sinus (CS) was noted. The length of CS in millimetres was measured from the union of great cardiac vein and left marginal vein up to the opening of the coronary sinus in the right atrium with vernier calliper. The shape of the coronary sinus ostium was noted & the measurements (length and breadth) of it were taken in millimetres with vernier calliper. Results: In 89.47% (34) specimens the coronary sinus was formed by the union of great cardiac vein and left marginal vein. -

A Core Syllabus for the Teaching of Gross Anatomy of the Thorax to Medical Students
This is an Open Access document downloaded from ORCA, Cardiff University's institutional repository: http://orca.cf.ac.uk/128413/ This is the author’s version of a work that was submitted to / accepted for publication. Citation for final published version: Moxham, Bernard J., Stephens, Shiby, Sharma, Deepak and Loukas, Marios 2020. A core syllabus for the teaching of gross anatomy of the thorax to medical students. Clinical Anatomy 33 (2) , pp. 300-315. 10.1002/ca.23522 file Publishers page: http://dx.doi.org/10.1002/ca.23522 <http://dx.doi.org/10.1002/ca.23522> Please note: Changes made as a result of publishing processes such as copy-editing, formatting and page numbers may not be reflected in this version. For the definitive version of this publication, please refer to the published source. You are advised to consult the publisher’s version if you wish to cite this paper. This version is being made available in accordance with publisher policies. See http://orca.cf.ac.uk/policies.html for usage policies. Copyright and moral rights for publications made available in ORCA are retained by the copyright holders. Clinical Anatomy (2019) MEDICAL AND DENTAL EDUCATION A Core Syllabus for the Teaching of Gross Anatomy of the Thorax to Medical Students 1,2 1 2 BERNARD J. MOXHAM , * SHIBY STEPHENS, DEEPAK SHARMA, AND MARIOS LOUKAS 2 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff, CF10 3AX, Wales, United Kingdom 2St George’s University, Grenada, West Indies Discussion is ongoing concerning the need to ensure the clinical relevance of the biomedical sciences. -

Human Anatomy Synopsis: Thorax, Abdomen, Pelvis
GERARD GORNIAK & WILLIAM CONRAD HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS Download free eBooks at bookboon.com 2 Human Anatomy Synopsis: Thorax, Abdomen, Pelvis 1st edition © 2018 Gerard Gorniak & William Conrad & bookboon.com ISBN 978-87-403-2213-2 Peer reviewers: Dr. Ed Kane, the University of St Augustine San Diego Dr. Hilmir Augustsson, University of St Augustine Miami Download free eBooks at bookboon.com 3 HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS CONTENTS CONTENTS Preface 7 1 Thoracic Cage 8 1.1 Boundaries 8 1.2 Osteology 8 1.3 Muscles of the Thorax 16 1.4 Intercostal Nerves (Fig. 1-13) 30 1.5 Intercostal Arteries and Veins (Figs. 1-13, 1-16, 1-17) 31 2 The Lungs 35 2.1 The Pleura (Fig. 2-2) 36 2.2 Lobes of the Lung (Figs 2-3, 2-4) 38 2.3 Pulmonary Vessels (Figs. 2-9, 2-10) 45 Free eBook on Learning & Development By the Chief Learning Officer of McKinsey Download Now Download free eBooks at bookboon.com Click on the ad to read more 4 HUMAN ANATOMY SYNOPSIS: THORAX, ABDOMEN, PELVIS CONTENTS 3 Heart 49 3.1 Mediastinum (Fig. 3-1) 49 3.2 Pericardium (Fig. 3-2) 51 3.3 Heart Overview (Fig. 3-3) 51 3.4 Structure of Arteries and Veins (Fig. 15-14) 67 4 Superior And Posterior Mediastina 72 4.1 Superior Mediastinum 72 4.2 Posterior Mediastinum 76 5 Abdominal Wall 84 5.1 Boundaries 84 5.2 Abdominal Planes (Table 4.1 and Fig. 4-1) 84 5.3 Anterior and Lateral Abdominal Walls 87 5.4 Inguinal Region (Figs. -

Instructions / સૂચના
AMD PROVISIONAL ANSWER KEY (CBRT) Name of The Post Professor, Anatomy, General State Service, Class-1 Advertisement No 41/2019-20 Preliminary Test Held On 16/02/2020 Que. No. 001-200 (Concerned Subject Only) Publish Date 17-02-2020 Last Date to Send 26-02-2020 Suggestion (S) Instructions / સૂચના Candidate must ensure compliance to the instructions mentioned below, else objections shall not be considered: - (1) All the suggestion should be submitted Physically in prescribed format of suggestion sheet. (2) Question wise suggestion to be submitted in the prescribed format of Suggestion Sheet published on the website. (3) All suggestions are to be submitted with reference to the Master Question Paper with provisional answer key, published herewith on the website. Objections should be sent referring to the Question, Question No. & options of the Master Question Paper. (4) Suggestions regarding question nos. and options other than provisional answer key (Master Question Paper) shall not be considered. (5) Objections and answers suggested by the candidate should be in compliance with the responses given by him in his answer sheet /response sheet. Objections shall not be considered, in case, if responses given in the answer sheet /response sheet and submitted suggestions are differed. For the purpose, the candidate shall attach a copy of his answersheet/ Response sheet along with his application(s). (6) Objection for each question shall be made on separate Suggestion sheet. Objection for more than one question in single Suggestion sheet shall not be considered & treated as cancelled. ઉમેદવાર ે નીચેની સૂચનાઓનું પાલન કરવાની તકેદારી રાખવી, અયથા વાંધા-સૂચન અંગે કર ેલ રજૂઆતો યાને લેવાશે નહીં (1) ઉમેદવારે વાંધા-સૂચનો િનયત કરવામાં આવેલ વાંધા-સૂચન પકથી રજૂ કરવાના રહેશે. -
Development of HEART 1-ATRIA
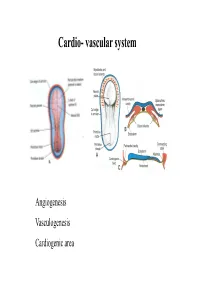
Cardio- vascular system Angiogenesis Vasculogenesis Cardiogenic area Formation of heart tube Myo-epicardial mantle Cardiac jelly Both heart tube and pericardial cavity develop from lateral plate mesoderm Primitive heart tube Occupies pericardial cavity Loop formation Chambers of primitive heart • Sinus venosus: central part & two horns Each horn receives- common cardinal Vs vitelline Vs Umbilical Vs • Common atrial chamber • Common ventricular chamber • Bulbous cordis: Three parts Proximal Middle conus cordis Distal truncus arteriosus Fate of sinus venosus Fate of sinus venosus • Left to right shunt of venous blood • Opening of sinus venosus is guarded by right & left sinuatrial valves • Left valve forms septum spurium • Right valve elongates, extends downwards and gets divided by the appearance of two sub- endocardial limbic bands – Crista terminalis – Valve of Inferior vena cava ( Eustachian valve) – Valve of coronary sinus (Thebsian valve) • Sinus venous gets absorbed into right atrium to form smooth part- sinus venarum Formation of inter atrial septum Formation of Interatrial Septum • Appearance of septum primum. • Appearance of atrio-ventricular endocardial cushions These cushions divide the AV canal into right and left. • Fusion of S. primum with endo-cardial cushion • Breakage of upper part of S. Primum • Appearance of S. Secundum • Formation of Foramen ovale in between septum primum & secundum • Valve of IVC directs blood towards left atrium through foramen ovale • After Birth: Pressure ↑ in left atrium Closure of foramen ovale Foramation of Fossa ovalis Annulus ovalis formed by septum secundum Pulmonary Veins • To begin- only one vein opening in to left atrium • First divides in to two and then both further divide to form four veins. -

Dr.Maher AL-Hadidi School of Medicine University of Jordan Spring 2019
Pericardium & HEART Dr.Maher AL-Hadidi School of Medicine University of Jordan Spring 2019 Spring 2019 Dr,Maher AL-Hadidi , University of Jordan 1 [Type here] Pericardium A double-walled fibroserous conical-shaped sac, inside middle mediastinum. Enclose the heart and roots of its large vessels. PericardiumVagus nerves Pericardium Pericardium SVC Pericardiophrenic A. Pericardiophrenic V. MusculophRt.renic Pericardiophrenic vessels branches Base [Type here] Diaphragm Diaphragm 2 Pericardium – Sagittal section Parts: 1 1. Fibrous pericardium. (outer) 2. Serous pericardium. 2 Fibrous pericardium (Inner) Conical-shaped fibrous sac. Base: Attached to central tendon of diaphragm. Layers . Apex: Attached to roots of large of heart vessels. Wall Prevent overextension of the Base heart. (potential) [Type here] Serous pericardium Complete serous sac invaginated by 1 the heart. Like pleura by the lung. (outer) 2 layers: 2 Parietal layer lines fibrous pericardium. (Inner) Visceral layer covers the heart as (Epicardium). Layers of heart Pericardial cavity: Wall A potential space between 2 serous Base layers. (potential) [Type here] Contents of pericardium: Superior vena cava Contents: Ascending Aorta Arch of aorta 1. Heart and all arteries, Transverse sinus Pulmonary veins and nerves. (separates arteries Artery from veins) 2 3 2. All Ascending aorta. 4 8 Left pulmonary 3. All Pulmonary Artery. 7 Right pulmonary vs. 9 6 4. Last 2cm of SVC. 1 5. Last 2cm of IVC. Serous pericardium + Oblique sinus 5 6. First part of the 4 diaphragm Fibrous pericardium pulmonary veins. Inferior vena cava 7. Transverse sinus. Thoracic aorta 8. Oblique sinus. Dr. Maher Hadidi, University of Jordan Heart Pyramidal-shaped muscular organ. SVC About the size of the person’s own fist.