A Case Study on Sal Mortality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
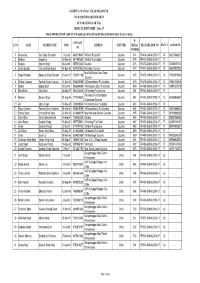
HSRT Final Result List of Phase Ii 2016-17
GARHWAL MANDAL VIKAS NIGAM LTD. 74/1 RAJPUR ROAD DEHRADUN HUNAR SE ROZGAR TAK RESULT LIST IN HSRT - 2016-17 FOOD PRODUCTION LIST OF PHASE II (01-07-16 TO 26-07-2016 AND 05-11-2016 To 20-11-2016) R- CONTACT S.NO NAME FATHER NAME DOB ADDRESS UNIT/TRHSERIAL REGISTRATION NO. RESULT AADHAR NO NO. NUMBER 1 Kusumlata Hari Singh Chaudhari 07-Jul-96 9456729647 Vill-Devli P.o-Ladoli Gauchar 474 FP-474-GMVN-2016-17 74 354216366072 2 Rakhee Jaspal Lal 20-May-93 8477042652 Vill-Kirult P.o-Gadola Gauchar 475 FP-475-GMVN-2016-17 72 - 3 Manisha Manvar Singh 04-Jun-95 9557012648 Gauchar Gauchar 476 FP-476-GMVN-2016-17 76 227426807142 4 Shanti Butola Charan Singh Butola 08-Nov-95 9557197933 Basantpur Gauchar Gauchar 477 FP-477-GMVN-2016-17 76 836603027938 Near By Bhadari Book Depo 5 Pooja Bhandari Bhagwat Singh Bhandari 15-Jan-97 7300811190 Gauchar 478 FP-478-GMVN-2016-17 74 371972974783 Gauchar 6 Shikha Chauhan Pushkar Singh Chauhan 18-Mar-94 9760578089 Vill-Bandarkhand P.o-Guachar Gauchar 479 FP-479-GMVN-2016-17 72 470447135945 7 Monika Brijpal Singh 28-Jun-96 9536644690 Vill-Kandwala Gaun P.o-Bhatoli Gauchar 480 FP-480-GMVN-2016-17 74 349874237370 8 Neha Bisht Vijay Bisht 04-May-97 9634304849 Vill-Vameda P.o-Gauchar Gauchar 481 FP-481-GMVN-2016-17 73 - Vill-Galnou P.o-Santi Sadan 9 Neelam Balveer Singh 03-Aug-96 8171144561 Gauchar 482 FP-482-GMVN-2016-17 75 535593948662 Chatwapipal Gauchar 10 Arti Uttam Singh 15-May-98 7088528388 Vill-Dhamdhama P.o-Bartoli Gauchar 483 FP-483-GMVN-2016-17 76 - 11 Rupa Chauhan Pushkar Singh Chauhan 06-Aug-96 9760578089 -

Effectiveness of Psychoeducation Program on Knowledge Among Adults of Selected Area of Dehradun, Uttarakhand
370 Indian Journal of Public Health Research & Development, May 2020, Vol. 11, No. 05 Effectiveness of Psychoeducation Program on Knowledge among Adults of Selected Area of Dehradun, Uttarakhand Rahul Singh Gusain1, Grace M. Singh2, Rajkumari Sylvia3 1M.Sc. Nursing Student, 2Associate Professor, Psychiatry and Mental Health Nursing Department, 3Associate Professor, Child Health Nursing Departement, Himalayan College of Nursing, Swami Rama Himalayan University, Dehradun, Uttarakhand, India Abstract Background: Mental health awareness delivers a wide range of knowledge and encompasses recognition and help-seeking behaviour. Mental health services should be assess at ground level to monitor its delivery. Different mean of communication can use to highlight the mental health issues. Methodology: Quantitative research approach with quasi pre-post-test control group design was adapted for present study. Total 126 adults were selected through systematic random sampling. The tools administered consisted of baseline data and structured knowledge questionnaire. Descriptive and inferential statistics used for analyses. Result: This study showed that the psychoeducation program on mental health awareness significantly improved in knowledge score from baseline mean 13.79 ±3.76 to 16.51±2.77; p<0.05 post intervention which was quantified by structured knowledge questionnaire. Conclusion: The study reported that psychoeducation program was effective in enhancing the awareness among adults. Keywords: Psychoeducation, mental health, awareness. Introduction revealed that stigma and discriminating to the mentally ill client is still present in the community.1 In a study The life cycle of the human being is a continuous in Tehran (2011) people reported that people suffering process, which consists of a series of development in from mental illness are discriminated and not accepted the human body. -

District Census Handbook, 1-Dehra Dun, Uttar Pradesh
:1 Census of India, 1951 DISTRICT CENSUS HANDBOOK UTTAR PRADESH I-DEHR.-\' DUN DISTRI("T ALLAHABAD: SUl'ElUN'IENDENT, PR1NTING AND STATIONERY, UTTAR PRAD~bH, INDIA 1954 DISTRICT CENSUS HANDBOOK 1951 DEHRA DUN DISTRICT FOREWORD Several States, including Uttar Pradesh, have been publishing village statistics by districts at each census. In 194I they were published in U. P. under the title "District Census Statistics" with a separate volume for each district. In the 1951 census, when the tabulation has been more elaborate than ever in view of the require ... ments of the country, the district ... wise volume has been expanded into a "District Census Handbook". which now contains the District Census Tables (furnishing data with break ... up for census tracts within the district), the District Index of Non... agricultural Occupations, agricultural statistics from 190V02 to 19.5°"'51 and other miscellaneous statistics in addition to the usual village population statistics. The village population statistics also are given in an elaborate form giving the division of the population among eight livelihood classes and other details. 2. It may be added here that a separate set of district ... wise volumes giving only population figures of rural areas by villages and of urban areas by wards and mohallas and entitled "District Population Statistics" has already been published. This separate series was necessitated by the urgent requirements of the U. P. Government for elections to local bodies. The printing of the District Census Handbooks involves colossal work and is bound to take some time. RAJESHW ARI PRASAD, IoA.S •• RAMPUIt: Superintendent, Census Operations, Septemher It 1954. -
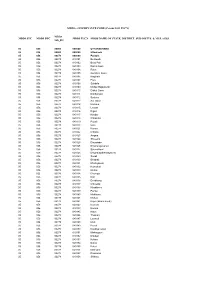
MDDS E-GOVERNANCE CODE (Census 2011 PLCN)
MDDS e-GOVERNANCE CODE (Census 2011 PLCN) MDDS MDDS STC MDDS DTC MDDS PLCN MDDS NAME OF STATE, DISTRICT, SUB-DISTTS. & VILLAGES Sub_DT 05 000 00000 000000 UTTARAKHAND 05 056 00000 000000 Uttarkashi 05 056 00278 000000 Puraula 05 056 00278 040101 Bestiwalli 05 056 00278 040102 Besti Palli 05 056 00278 040103 Rama Gaon 05 056 00278 040104 Raun 05 056 00278 040105 Gundiyar Gaon 05 056 00278 040106 Nagjhala 05 056 00278 040107 Pora 05 056 00278 040108 Sukdala 05 056 00278 040109 Moltari Rajputonki 05 056 00278 040110 Dhikal Gaon 05 056 00278 040111 Dokhariyani 05 056 00278 040112 Syaluka 05 056 00278 040113 Sar Gaon 05 056 00278 040114 Kaslaun 05 056 00278 040115 Lewtari 05 056 00278 040116 Digari 05 056 00278 040117 Kimdar 05 056 00278 040118 Chhanika 05 056 00278 040119 Paunti 05 056 00278 040120 Gaul 05 056 00278 040121 Kurara 05 056 00278 040122 Chhara 05 056 00278 040123 Angora 05 056 00278 040124 Shreekot 05 056 00278 040125 Dhundada 05 056 00278 040126 Dhyoralagasunali 05 056 00278 040127 Suranukiseri 05 056 00278 040128 Dhyoralagakhadkyasem 05 056 00278 040129 Sunali 05 056 00278 040130 Bhadrali 05 056 00278 040131 Kharkyasem 05 056 00278 040132 Kumarkot 05 056 00278 040133 Moltari 05 056 00278 040134 Dhampur 05 056 00278 040135 Koti 05 056 00278 040136 Devdhung 05 056 00278 040137 Chhiwala 05 056 00278 040138 Khablisera 05 056 00278 040139 Purola 05 056 00278 040140 Makhana 05 056 00278 040141 Molkat 05 056 00278 040142 Pujeli (Brahmanoki) 05 056 00278 040143 Kumola 05 056 00278 040144 Korana 05 056 00278 040145 Nauri 05 056 00278 040146 Thakrari 05 056 00278 040147 Lamkoti 05 056 00278 040148 Math 05 056 00278 040149 Puseli 05 056 00278 040150 Kandiyal Gaon 05 056 00278 040151 Mahargaon 05 056 00278 040152 Dokhari 05 056 00278 040153 Kufara 05 056 00278 040154 Dukra 05 056 00278 040155 Rateri 05 056 00278 040156 Mairiyara 05 056 00278 040157 Dhakaraa 05 056 00278 040158 Shikaru MDDS e-GOVERNANCE CODE (Census 2011 PLCN) MDDS MDDS STC MDDS DTC MDDS PLCN MDDS NAME OF STATE, DISTRICT, SUB-DISTTS. -

Remote Sensing and Geographic Information System (RS&GIS)
PILOT PROJECT ABSTRACT Remote Sensing and Geographic Information System (RS & GIS) PG Course (Phase-I) First PG Course in RS & GIS (April 1996-December 1996) Development of Spatial Decision Support System for optimum location of additional Optimum land use planning by Remote Sensing & GIS techniques village amenities Supervisors Mr. Jo IL Gwang Mr. L.M. Pande, ASD, Supervisors DPR Korea Dr. Jitendra Prasad, ASD Mr. Iftikhar Uddin Sikder Dr. K.P. Sharma, RRSSC-D, IIRS, Dehradun, India Bangladesh Dr. A.P. Subudhi, HUSAG Dr. P.S. Roy, FED IIRS, Dehradun, India sing multisspectral data, it was attempted to prepare thematic maps of a region. UFurther, Iand evaluation was made using standard techniques to assist in an optimum he study intended to develop a Spatial Decision Support System (SDSS) to aid decision landuse planning and land utilization scheme for the region. Tmakers to plan in spatial context, especially with respect to village ammenities. Various parameters like human settlement, service population, distance factor of the farthest settlement from any of the service cantre were used as indicators to derive at the optimum location of amenities. Emphasis was laid on location of hospitals. The System offers a menu based interface for the prospective planner. Development of Spatial Decision Support System for optimum location of additional Watershed prioritization using remote sensing, GIS and AGNPS model village amenities Supervisor Mr. Hong Yong IL Mr. P.L.N. Raju, GID Supervisor DPR Korea IIRS, Dehradun, India Ms. Nagma Yasmin Mr. R.C. Lakhera, GSD Bangladesh IIRS, Dehradun, India sing multispectral remote sensing data & topographic maps, estimating of runoff and soil loss were attempted. -

Curriculum Vitae
CURRICULUM VITAE Dr. Suniti Kumar Kuriyal Old Ghamandpur Road Kanharwala, Bhaniyawala Dehradun, U.K. – 248144 E-mail : [email protected] Mobile No : 9456556249 PERSONAL DETAILS Name : Dr. Suniti Kumar Kuriyal Father’s Name : Shri Dhiraj Mani Kuriyal Date of Birth : 12th June 1967 Language Known : Hindi, English Nationality : Indian Category : General Hobbies : Gardening Permanent Address : Village – Sukrey, P.O. Thapla Distt - Tehri Garhwal Uttarakhand EDUCATIONAL QUALIFICATION S.No Exam Passed Board/Univ Year Percentage Subject 1. B.Sc. Rohilkhand 1985 60.00 Zoology, University, Bareilly Botany, Chemistry 2. M.Sc. Rohilkhand 1987 60.01 Botany Univeristy Bareilly 3 Ph.D. Rohilkhand 1992 - Botany University, Bareilly Present Status : Assistant Professor (Botany) Govt. Post Graduate College, Doiwala, Dehradun. U.K. EXPERIENCE Teaching : 16 years P.G. Classes 20 years U.G. Classes Temporary Lecturer (Environmental Science) 1994-95, Department of Environmental Science, Bareilly College, Bareilly (U.P.) Temporary Lecturer (Botany) 1995 – June 2001. P.G. Department of Botany, Bareilly College, Bareilly (U.P.) Visiting Lecturer (Botany) 2001 to 28th Oct. 2010, P.G. Department of Botany, Govt P.G. College, Uttarakashi (U.K.) Visiting Lecturer (Botany) 29th Oct. 2010 to 17th March 2013, Department of Botany, Govt. Degree College Doiwala, Dehradun, U.K. Research : More then 18 years PROFESSIONAL EXPERIENCE Biotechnology Consultant : Directorate of Industries Kanpur (U.P.) Senior Biotech Consultant : SRV Floritech & Consultancy Bareilly (U.P.) Mushroom Consultant : Koshika Training Centre, Bareilly (U.P.) Guest Faculty (1995-2001) : P.G. Diploma in Professional Biotechnology , Bareilly College, Bareilly (U.P.) B.Sc. Biotechnology, Bareilly College, Bareilly (U.P.) B.Sc. Environmental Science, Bareilly College, Bareilly (U.P.) Diploma in Tissue Culture and Floriculture, Bareilly College, Bareilly (U.P.) Guest Faculty (2004-2008) Diploma in Forestry and Wild Life Management, Govt. -

1 Rapid Mapping Exercise (RME) of Medicinal and Aromatic Plants
Rapid Mapping Exercise (RME) of Medicinal and Aromatic Plants (MAPs): The Uttarakhand Experience B.S. Adhikari & G.S. Rawat Department of Habitat Ecology, Wildlife Institute of India, P.O. Box 18, Chandrabani, Dehradun, Uttarakhand Email: [email protected] Genesis The Uttarakhand Forest Department is vested with the larger responsibility of managing forests (71%) under various categories viz., Protected Areas (PAs), Reserved Forests (RFs), Protected Forests (PFs) and un-classified forests for biodiversity conservation and ecosystem services (both tangible and intangible), meeting the biomass demands of the local communities and a variety of other functions. The department has been proactive in the conservation and development of Medicinal and aromatic plants (MAPs) in the state. Major activities pertaining to this sector include control of illegal harvest of MAPs from the Reserved Forests, protection of natural habitats, development of MAP nurseries, inventory and monitoring of MAPs, promoting MAP plantation under afforestation and reforestation drives. The department has also taken steps to control illegal harvest of MAPs from wild. To implement this, the MAP species have been grouped into three categories viz., (i) species banned for collection (ii) species open for collection, and (iii) species which can be sustainably harvested. In recent years the department has given special emphasis on incorporating Non Timber Forest Produce (NTFP)/MAPs in forestry Working Plans, especially for those forest divisions, which fall under non-forested areas such as alpine grasslands, rocky and drier tracts which otherwise do not support forest vegetation and are congenial for the growth of a variety of MAPs. The state of Uttarakhand has been projected as ‘Herbal State’ of India. -

View of Consolidated Abstract Prescribed in Art.19 of the Account Code, Volume IV Has Been Done by SO?
REPORT ID: TC4615 OFFICE OF THE ACCOUNTANT GENERAL PRINTED BY: AAO2 Draft PRINTED ON: 25/08/2020 16:58:17 Consolidated Abstract Month of Account: 01/06/2020 Major Head: 2054 Treasury and Accounts Administration Grant Number: 07 Plan / Non Plan: N REPORT ID: TC4615 OFFICE OF THE ACCOUNTANT GENERAL PRINTED BY: AAO2 Draft PRINTED ON: 25/08/2020 16:58:17 Consolidated Abstract Head of Account Budget Provision Current Month Progressive 2054 00 003 03 00 56 Voted 3,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 3,00,00,000.00 .00 .00 Total: 00 Voted 3,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 3,00,00,000.00 .00 .00 Total: 03 Voted 3,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 3,00,00,000.00 .00 .00 97 01 56 Voted 60,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 60,00,00,000.00 .00 .00 Total: 01 Voted 60,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 60,00,00,000.00 .00 .00 Total: 97 Voted 60,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 60,00,00,000.00 .00 .00 Total: 003 Voted 63,00,00,000.00 .00 .00 Charged .00 .00 .00 Total 63,00,00,000.00 .00 .00 095 01 02 04 Voted 10,000.00 .00 .00 Charged .00 .00 .00 Total 10,000.00 .00 .00 10 Voted 5,00,000.00 .00 .00 Charged .00 .00 .00 Total 5,00,000.00 .00 .00 27 Voted 50,00,000.00 .00 .00 Charged .00 .00 .00 Total 50,00,000.00 .00 .00 42 Voted 2,00,000.00 .00 .00 Charged .00 .00 .00 Total 2,00,000.00 .00 .00 Total: 02 Voted 57,10,000.00 .00 .00 Charged .00 .00 .00 Total 57,10,000.00 .00 .00 03 04 Voted 1,000.00 .00 .00 Charged .00 .00 .00 Total 1,000.00 .00 .00 10 Voted 1,000.00 .00 .00 Charged .00 .00 .00 Total 1,000.00 .00 .00 26 Voted 1,000.00 .00 .00 Charged .00 .00 .00 Total 1,000.00 .00 .00 Page No. -

Doiwala(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in Which the Assembly Constituency Is Located: 5-Hardwar(GEN) Electors
ELECTORAL ROLL - 2017 STATE - (S28) UTTARAKHAND No., Name and Reservation Status of Assembly Constituency: 23-Doiwala(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in which the Assembly Constituency is located: 5-Hardwar(GEN) Electors 1. DETAILS OF REVISION Year of Revision : 2017 Type of Revision : De-novo preparation Qualifying Date : 01.01.2017 Date of Draft Publication: 04.10.2017 2. SUMMARY OF SERVICE ELECTORS A) NUMBER OF ELECTORS 1. Classified by Type of Service Name of Service No. of Electors Members Wives Total A) Defence Services 1472 8 1480 B) Armed Police Force 0 0 0 C) Foreign Service 1 0 1 Total in Part (A+B+C) 1473 8 1481 2. Classified by Type of Roll Roll Type Roll Identification No. of Electors Members Wives Total I Original Preliminary Preliminary De-novo 1473 8 1481 Roll, 2017 preparation of last part of Electoral Roll Net Electors in the Roll 1473 8 1481 Elector Type: M = Member, W = Wife Page 1 Draft Electoral Roll, 2017 of Assembly Constituency 23-Doiwala (GEN), (S28)UTTARAKHAND A . Defence Services Sl.No Name of Elector Elector Rank Husband's Regimental Address for House Address Type Sl.No. despatch of Ballot Paper (1) (2) (3) (4) (5) (6) (7) Assam Rifles 1 MANBIR SINGH M Naib Headquarters Directorate General UTTRANCHAL ENCLAVE Subedar Assam Rifles, Record Branch, SECTOR-I HARIPUR Laitumkhrah,Shillong-793011 DEHRADUN DEHRADUN NAWADA 248005 Dehradun 2 VIKRAM SINGH M Rifleman Headquarters Directorate General NAKRONDA Assam Rifles, Record Branch, DEHRADUN DEHRADUN Laitumkhrah,Shillong-793011 -

Mortality in the Protected Leopard's Population, Uttarakhand, North India
International Journal of Ecosystem 2012, 2(4): 44-53 DOI: 10.5923/j.ije.20120204.01 Mortality in the Protected Leopard’s Population, Uttarakhand, North India: A Free-Ranging Wildlife Species in Threat Ritesh Joshi1,*, Rajendra Agarwal2 1Conservation and Survey Division, Ministry of Environment and Forests, Govt. of India, Paryavaran Bhawan, CGO Complex, New Delhi–110 003, India 2Wildlife Protection Society of India, Uttarakhand Region, Kankhal, Haridwar, 249 401, Uttarakhand, India Abstract Large cats are vulnerable to local extinction in fragmented landscapes mainly due to large scope developmental and anthropogenic activities. Present study highlights the mortality of protected leopard’s population in Uttarakhand state, north-west India. In between January 2009 to October 2010, 78 leopards have died due to various reasons accounted pri- marily for unnatural deaths. Maximum deaths occurred in between February 2009 & April 2010 and notably 37 leopards died since January, 2010. The mortality rate for females was significantly higher than for males. Notably, 11 leopards were found dead scrambled in trap and some deaths occurred while providing treatment after rescuing the animal. In addition, 21 cases of leopard’s poaching (illegal wildlife trade) were also documented in between January 2009 to March 2010 in which 35 leopard’s skins were recovered, which highlighted that poaching is also ongoing in some remote areas. Status of man-animal conflict in Uttarakhand is severely increasing; in between November 2000 to December 2008, 180 people died in leopard’s attack, whereas 343 were injured during encounters and leopard’s attacks. On the other hand, 394 leopards died due to other reasons during the said period and 50 were declared as man-eater, which were shot dead or translocated to other protected habitat. -
Registration Detail for Film Appreciation Course Dehadun
Registration Detail For Film Appreciation Course Dehadun Sr.No. Name नाम Organization Date of Address Contact No. e-mail.com Birth 1 Ananya Rawat अना रावत Student 25 -9-1992 c-2/5, New type III, Ordnance 9971992012 [email protected] Factory Estate, Raipur Dehradun 2 Mohan lal मोहन लाल Haryana School 8-6-1981 Mohan lal s/o Shri. Om Prakash 9466729957 [email protected] Education V.P.O.Rattakhera, deptt- The.Ratia,Distt.Fatehabad, Haryana 3 Suraj Kumar सूरज कुमार Student 1997 A-1, Sector -3, Vasant Vishar, New 9045717023 [email protected] Tehri, Tehri Garhwal m 4 Manuj Kumar Tyagi मनुज कुमार ागी Film maker 1-1-1978 Rajeshwar Nagar phase5, 9897546564 manujt [email protected] Sahastradhara Road, Dehradun, Behind PNB 5 Rahul Gupta राल गुा Teacher 12 -12 -1987 14/275 Mandi Saeed khan agra - 8979194912 [email protected] 282002 6 Abhisheek Thalwal अिभषेक थलवाल Student (Mass 5-10 -1998 H.No.141,Lane -4, Block -B, Upper 8650034986 [email protected] Comm) Rajiv Nagar, Dharampur Danda, m Dehradun 7 Sachin Singh Rawat सिचन िसंह रावत Student 19 -12 -1998 D-125, Nehru Colony Dharampur, 8979621550 [email protected] Dehradun 8 Shweta Bhandari ेता भारी Student 31 -10 -1998 Shridev Suman Colony lane - 8449588745 [email protected] 2,Shamshergarh Road, Balawala, om Dehradun 9 Deepak Kumar दीपक कुमार Under 10 -6-1977 Deptt. Of Finance, Room No. -2, 9927699143 [email protected] Secretary, West Block, 04 Subhas Road, Uttarakhand uttarakhand Sachivalaya, Govt. -

S Name of Beneficiary Full Address (Vill, Block, Distric
Page 1 Annexure - XIII National Biogas and Manure Management Programme 1 Name of State/UT-Uttarakhand 2 Name of the SND/SNA/KVIC of the State - Rural Development Department 3 District - Dehradun 4 Report on website for the year - 2018-19 5 Total nos. of Biogas Plants reported as completed in all respect - 116 6 Date of Putting the list on website - 7 Particulars of the beneficiaries viz : Date of commisioningof plant Biogas of Date payment linked with Aadhaar number paymentAadhaar linkedwith basis if yes, name and address of turnof andaddress name yes, if basis Name of the field supervising official official supervising field the of Name of the SND/SNA/KVIC the of who verified ……. MNRE/State Subsidy/Subsidy MNRE/State ……. disbursed abd dilivery mechanism of mechanism abddisbursed dilivery identification mark/code number mark/code on identification Size and model of biogas plant andand modelbiogasplant of Size Wether plants installed on turnkey installed plants Wether Photograph of the beneficiary with Photographbeneficiary the of Whether ISI marked biogas stove. biogasstove. marked ISI Whether Amount subsidyofRs.) (in paid the completion of biogasplantcompletionof the Date and amount subsidyof Date key agency/ worker/ RET worker/ keyagency/ No.s of family members family ofNo.s Nos. of Cattles Owned Cattles ofNos. ProvidedYES/No Category biogasplants the Full address plant Biogas the of the Father's (Vill, block, Beneficia S.No Name/Husband District and Block Year Beneficiary Name ry . 's Name of State) and General/ beneficiary contact