High Performance Ozone Based Advanced Oxidation Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Abiogenesis
Historical Development of the Distinction between Bio- and Abiogenesis. Robert B. Sheldon NASA/MSFC/NSSTC, 320 Sparkman Dr, Huntsville, AL, USA ABSTRACT Early greek philosophers laid the philosophical foundations of the distinction between bio and abiogenesis, when they debated organic and non-organic explanations for natural phenomena. Plato and Aristotle gave organic, or purpose-driven explanations for physical phenomena, whereas the materialist school of Democritus and Epicurus gave non-organic, or materialist explanations. These competing schools have alternated in popularity through history, with the present era dominated by epicurean schools of thought. Present controversies concerning evidence for exobiology and biogenesis have many aspects which reflect this millennial debate. Therefore this paper traces a selected history of this debate with some modern, 20th century developments due to quantum mechanics. It ¯nishes with an application of quantum information theory to several exobiology debates. Keywords: Biogenesis, Abiogenesis, Aristotle, Epicurus, Materialism, Information Theory 1. INTRODUCTION & ANCIENT HISTORY 1.1. Plato and Aristotle Both Plato and Aristotle believed that purpose was an essential ingredient in any scienti¯c explanation, or teleology in philosophical nomenclature. Therefore all explanations, said Aristotle, answer four basic questions: what is it made of, what does it represent, who made it, and why was it made, which have the nomenclature material, formal, e±cient and ¯nal causes.1 This aristotelean framework shaped the terms of the scienti¯c enquiry, invisibly directing greek science for over 500 years. For example, \organic" or \¯nal" causes were often deemed su±cient to explain natural phenomena, so that a rock fell when released from rest because it \desired" its own kind, the earth, over unlike elements such as air, water or ¯re. -

Viscosity of Gases References
VISCOSITY OF GASES Marcia L. Huber and Allan H. Harvey The following table gives the viscosity of some common gases generally less than 2% . Uncertainties for the viscosities of gases in as a function of temperature . Unless otherwise noted, the viscosity this table are generally less than 3%; uncertainty information on values refer to a pressure of 100 kPa (1 bar) . The notation P = 0 specific fluids can be found in the references . Viscosity is given in indicates that the low-pressure limiting value is given . The dif- units of μPa s; note that 1 μPa s = 10–5 poise . Substances are listed ference between the viscosity at 100 kPa and the limiting value is in the modified Hill order (see Introduction) . Viscosity in μPa s 100 K 200 K 300 K 400 K 500 K 600 K Ref. Air 7 .1 13 .3 18 .5 23 .1 27 .1 30 .8 1 Ar Argon (P = 0) 8 .1 15 .9 22 .7 28 .6 33 .9 38 .8 2, 3*, 4* BF3 Boron trifluoride 12 .3 17 .1 21 .7 26 .1 30 .2 5 ClH Hydrogen chloride 14 .6 19 .7 24 .3 5 F6S Sulfur hexafluoride (P = 0) 15 .3 19 .7 23 .8 27 .6 6 H2 Normal hydrogen (P = 0) 4 .1 6 .8 8 .9 10 .9 12 .8 14 .5 3*, 7 D2 Deuterium (P = 0) 5 .9 9 .6 12 .6 15 .4 17 .9 20 .3 8 H2O Water (P = 0) 9 .8 13 .4 17 .3 21 .4 9 D2O Deuterium oxide (P = 0) 10 .2 13 .7 17 .8 22 .0 10 H2S Hydrogen sulfide 12 .5 16 .9 21 .2 25 .4 11 H3N Ammonia 10 .2 14 .0 17 .9 21 .7 12 He Helium (P = 0) 9 .6 15 .1 19 .9 24 .3 28 .3 32 .2 13 Kr Krypton (P = 0) 17 .4 25 .5 32 .9 39 .6 45 .8 14 NO Nitric oxide 13 .8 19 .2 23 .8 28 .0 31 .9 5 N2 Nitrogen 7 .0 12 .9 17 .9 22 .2 26 .1 29 .6 1, 15* N2O Nitrous -

Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbon Ions
J. Phys. Chem. A 2000, 104, 3655-3669 3655 Infrared Spectroscopy of Matrix-Isolated Polycyclic Aromatic Hydrocarbon Ions. 5. PAHs Incorporating a Cyclopentadienyl Ring† D. M. Hudgins,* C. W. Bauschlicher, Jr., and L. J. Allamandola NASA Ames Research Center, MS 245-6, Moffett Field, California 94035 J. C. Fetzer CheVron Research Company, Richmond, California 94802 ReceiVed: NoVember 5, 1999; In Final Form: February 9, 2000 The matrix-isolation technique has been employed to measure the mid-infrared spectra of the ions of several polycyclic aromatic hydrocarbons whose structures incorporate a cyclopentadienyl ring. These include the cations of fluoranthene (C16H10), benzo[a]fluoranthene, benzo[b]fluoranthene, benzo[j]fluoranthene, and benzo- [k]fluoranthene (all C20H12 isomers), as well as the anions of benzo[a]fluoranthene and benzo[j]fluoranthene. With the exception of fluoranthene, which presented significant theoretical difficulties, the experimental data are compared to theoretically calculated values obtained using density functional theory (DFT) at the B3LYP/ 4-31G level. In general, there is good overall agreement between the two data sets, with the positional agreement between the experimentally measured and theoretically predicted bands somewhat better than that associated with their intensities. The results are also consistent with previous experimental studies of polycyclic aromatic hydrocarbon ions. Specifically, in both the cationic and anionic species the strongest ion bands typically cluster in the 1450 to 1300 cm-1 range, reflecting an order-of-magnitude enhancement in the CC stretching and CH in-plane bending modes between 1600 and 1100 cm-1 in these species. The aromatic CH out-of- plane bending modes, on the other hand, are usually modestly suppressed (e 2x - 5x) in the cations relative to those of the neutral species, with the nonadjacent CH modes most strongly affected. -

Measurement of Cp/Cv for Argon, Nitrogen, Carbon Dioxide and an Argon + Nitrogen Mixture
Measurement of Cp/Cv for Argon, Nitrogen, Carbon Dioxide and an Argon + Nitrogen Mixture Stephen Lucas 05/11/10 Measurement of Cp/Cv for Argon, Nitrogen, Carbon Dioxide and an Argon + Nitrogen Mixture Stephen Lucas With laboratory partner: Christopher Richards University College London 5th November 2010 Abstract: The ratio of specific heats, γ, at constant pressure, Cp and constant volume, Cv, have been determined by measuring the oscillation frequency when a ball bearing undergoes simple harmonic motion due to the gravitational and pressure forces acting upon it. The γ value is an important gas property as it relates the microscopic properties of the molecules on a macroscopic scale. In this experiment values of γ were determined for input gases: CO2, Ar, N2, and an Ar + N2 mixture in the ratio 0.51:0.49. These were found to be: 1.1652 ± 0.0003, 1.4353 ± 0.0003, 1.2377 ± 0.0001and 1.3587 ± 0.0002 respectively. The small uncertainties in γ suggest a precise procedure while the discrepancy between experimental and accepted values indicates inaccuracy. Systematic errors are suggested; however it was noted that an average discrepancy of 0.18 between accepted and experimental values occurred. If this difference is accounted for, it can be seen that we measure lower vibrational contributions to γ at room temperature than those predicted by the equipartition principle. It can be therefore deduced that the classical idea of all modes contributing to γ is incorrect and there is actually a „freezing out‟ of vibrational modes at lower temperatures. I. Introduction II. Method The primary objective of this experiment was to determine the ratio of specific heats, γ, for gaseous Ar, N2, CO2 and an Ar + N2 mixture. -

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

Absorption and Laser Induced Fluorescence Spectroscopy Of
Astronomy in Focus, Volume 1, Focus Meeting 12, E17 XXIXth IAU General Assembly, August 2015 c International Astronomical Union 2017 Piero Benvenuti, ed. doi:10.1017/S1743921317005075 Absorption and Laser Induced Fluorescence Spectroscopy of Neutral Polycyclic Aromatic Hydrocarbons in Argon matrices Salma Bejaoui 1,2, Farid Salama 1 and Ella Sciamma-O’Brien 1,3 1 NASA Ames Research Center, Mail Stop 245-6, Moffett Field, California 94035-1000 2 NPP, Oak Ridge Associated Universities 3 BAER Institute, Petaluma, CA email: [email protected] Keywords. PAH, fluorescence, UV-VIS, ERE, comet. Polycyclic aromatic hydrocarbons (PAHs) are considered as plausible carriers for the extended red emission (ERE), a photoluminescent process associated with a wide variety of interstellar environments, as well as for broad emission band features seen in cometary spectra. We report the absorption spectra of phenanthrene, anthracene, fluoranthene, pentacene, pyrene, chrysene and triphenylene isolated at 10 K in solid argon matrices together with laser induced fluores- cence (LIF) spectra at 355 nm of matrix-isolated anthracene and fluoranthene. LIF spectra are compared with the UV/blue fluorescence spectra of the Red Rectangle Nebula (RR). The LIF spectra measured in solid Ar matrices have been shifted to the predicted position of the PAH band emission in the gas phase for comparison with the astronomical observations (Fig. 1). These preliminary results indicate that small neutral PAHs can well account for the blue fluorescence observed in the RR as it has been previously proposed (Vijh, et al. (2004)). LIF spectra of anthracene measured in Ar matrices are also compared to the emission spectra of 1P/Halley’s inner coma(Fig. -

Hydrogen, Nitrogen and Argon Chemistry
Hydrogen, Nitrogen and Argon Chemistry Author: Brittland K. DeKorver Institute for Chemical Education and Nanoscale Science and Engineering Center University of Wisconsin-Madison Purpose: To learn about 3 of the gases that are in Earth’s atmosphere. Learning Objectives: 1. Understand that the atmosphere is made up of many gases. 2. Learn about the differences in flammability of nitrogen and hydrogen. 3. Learn what is produced during electrolysis. Next Generation Science Standards (est. 2013): PS1.A: Structure and Properties of Matter PS1.B: Chemical Reactions PS3.D: Energy in Chemical Processes and Everyday Life (partial) ETS1.A: Defining Engineering Problems ETS1.B: Designing Solutions to Engineering Problems ETS1.C: Optimizing the Design Solution National Science Education Standards (valid 1996-2013): Physical Science Standards: Properties and changes of properties in matter Earth and Space Science Standards: Structure of the earth system Suggested Previous Activities: Oxygen Investigation and Carbon Dioxide Chemistry Grade Level: 2-8 Time: 1 hour Materials: Safety glasses Manganese dioxide Work gloves (for handling Tea-light candles steel wool) Matches Test tubes Spatulas Beakers Wooden splints 100mL graduated cylinders Waste bucket for burnt 15mL graduated cylinders materials 3% hydrogen peroxide Vinegar Baking soda Safety: 1. Students should wear safety glasses during all activities. 2. Students should not be allowed to use matches or candle. 3. Students should be cautioned to only mix the chemicals as directed. Introduction: Air refers to the mixture of gases that make up the Earth’s atmosphere. In this lesson, students will learn about hydrogen (present in very, very small amounts in our atmosphere), nitrogen (the most prevalent gas), and argon (third behind nitrogen and oxygen.) Procedures: 1. -
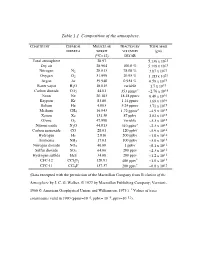
Table 1.1 Composition of the Atmosphere
Table 1.1 Composition of the atmosphere. CONSTITUENT CHEMICAL MOLECULAR FRACTION BY TOTAL MASS FORMULA WEIGHT VOLUME IN (gm) (12C=12) DRY AIR Total atmosphere 28.97 5.136 x 1021 Dry air 28.964 100.0 % 5.119 x 1021 Nitrogen N2 28.013 78.08 % 3.87 x 1021 Oxygen O2 31.999 20.95 % 1.185 x 1021 Argon Ar 39.948 0.934 % 6.59 x 1019 Water vapor H2O 18.015 variable 1.7 x 1019 Carbon dioxide CO2 44.01 353 ppmv* ~2.76 x 1018 Neon Ne 20.183 18.18 ppmv 6.48 x 1016 Krypton Kr 83.80 1.14 ppmv 1.69 x 1016 Helium He 4.003 5.24 ppmv 3.71 x 1015 Methane CH4 16.043 1.72 ppmv* ~4.9 x 1015 Xenon Xe 131.30 87 ppbv 2.02 x 1015 Ozone O3 47.998 variable ~3.3 x 1015 Nitrous oxide N2O 44.013 310 ppbv* ~2.3 x 1015 Carbon monoxide CO 28.01 120 ppbv ~5.9 x 1014 Hydrogen H2 2.016 500 ppbv ~1.8 x 1014 Ammonia NH3 17.03 100 ppbv ~3.0 x 1013 Nitrogen dioxide NO2 46.00 1 ppbv ~8.1 x 1012 Sulfur dioxide SO2 64.06 200 pptv ~2.3 x 1012 Hydrogen sulfide H2S 34.08 200 pptv ~1.2 x 1012 * 13 CFC-12 CCl2F2 120.91 480 pptv ~1.0 x 10 * 12 CFC-11 CCl3F 137.37 280 pptv ~6.8 x 10 (Data excerpted with the permission of the Macmillan Company from Evolution of the Atmosphere by J. -

SDS # : 002051 Supplier's Details : Airgas USA, LLC and Its Affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253
SAFETY DATA SHEET Nonflammable Gas Mixture: Argon 90-99.9999% / Methane 1ppm-10% (P-5 & P-10) Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Argon 90-99.9999% / Methane 1ppm-10% (P-5 & P-10) Other means of : Not available. identification Product type : Gas. Product use : Synthetic/Analytical chemistry. SDS # : 002051 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation. -

Effect of Argon-Nitrogen Mixing Gas During Magnetron Sputtering on Titanium Interlayer
Rev.Adv.Mater.Sci.80 16(2007) 80-87N. Panich, P. Wangyao, S. Hannongbua, P. Sricharoenchai and Y. Sun EFFECT OF ARGON-NITROGEN MIXING GAS DURING MAGNETRON SPUTTERING ON TITANIUM INTERLAYER DEPOSITION WITH TiB2 COATINGS ON HIGH SPEED STEEL N. Panich1, P. Wangyao1, S. Hannongbua1, P. Sricharoenchai2 and Y. Sun3 1 Metallurgy and Materials Science Research Institute, Chulalongkorn University, Phrayathai Rd., Pathumwan, Bangkok, 10330, Thailand 2Dept. of Metallurgical Engineering, Faculty of Engineering, Chulalongkorn University, Phrayathai Rd., Pathumwan, Bangkok, 10330, Thailand 3 School of Engineering & Technology, De Montfort University, Leicester LE1 9BH, UK Received: July 14, 2007 Abstract. It has been a common practice that in the deposition of a ceramic coating onto a sub- strate, a thin titanium interlayer is used to improve the adhesion between the coating and the substrate. But the structure and strength of the interlayer play an important role in determining the adhesion strength of the coating. This work involves the deposition of titanium diboride (TiB2) based nanostructured coatings on high speed steel substrates. A titanium interlayer was applied by sputtering a titanium target in pure argon atmosphere and in argon-nitrogen gas mixtures to affect nitrogen doping of the interlayer. Structural and properties analysis revealed that various contents of nitrogen gas in argon-nitrogen gas mixture during deposition by magnetron sputtering has significant effect on the adhesion between the TiB2 coating and the high speed steel substrate. There exists an optimum degree of nitrogen incorporation in the interlayer that results in the maxi- mum enhancement in adhesion. The beneficial effect of nitrogen gas mixing is discussed in terms of the modified structure and increased hardness of the interlayer, which provides stronger sup- port for mechanical loading. -

Gas Mixture (Argon Balance, Carbon Dioxide <10%, Oxygen 0.1
Gas Mixture (Argon Balance, Carbon Dioxide <10%, Oxygen 0.1ppm - 16.94%) Safety Data Sheet P-6304 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/2005 Revision date: 10/27/2016 Supersedes: 09/01/2015 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Mixture Name : Gas Mixture (Argon Balance, Carbon Dioxide <10%, Oxygen 0.1ppm - 16.94%) Other means of identification : Stargon Gas Blends Robostar CS 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Electric Arc Welding Industrial use 1.3. Details of the supplier of the safety data sheet Praxair, Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA T 1-800-772-9247 (1-800-PRAXAIR) - F 1-716-879-2146 www.praxair.com 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS-US classification Compressed gas H280 2.2. Label elements GHS-US labeling Hazard pictograms (GHS-US) : GHS04 Signal word (GHS-US) : WARNING Hazard statements (GHS-US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION CGA-HG01 - MAY CAUSE FROSTBITE CGA-HG03 - MAY INCREASE RESPIRATION AND HEART RATE Precautionary statements (GHS-US) : P202 - Do not handle -

SDS # : 002004 Supplier's Details : Airgas USA, LLC and Its Affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253
SAFETY DATA SHEET Nonflammable Gas Mixture: Argon 1ppm-98% / Carbon Dioxide 2-99.9999% Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Argon 1ppm-98% / Carbon Dioxide 2-99.9999% Other means of : Not available. identification Product type : Gas. Product use : Synthetic/Analytical chemistry. SDS # : 002004 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. May increase respiration and heart rate. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation.