First Observation of Atomic Levels for the Element Fermium (Z ˆ 100)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Genius of the Periodic Table
GENIUS OF THE PERIODIC TABLE "Isn't it the work of a genius'. " exclaimed Academician V.I. Spitsyn, USSR, a member of the Scientific Advisory Committee when talking to an Agency audience in January. His listeners shared his enthusiasm. Academician Spitsyn was referring to the to the first formulation a hundred years ago by Professor Dmitry I. Mendeleyev of the Periodic Law of Elements. In conditions of enormous difficulty, considering the lack of data on atomic weights of elements, Mendeleyev created in less than two years work at St. Petersburg University, a system of chemical elements that is, in general, still being used. His law became a powerful instrument for further development of chemistry and physics. He was able immediately to correct the atomic weight numbers of some elements, including uranium, whose atomic weight he found to be double that given at the time. Two years later Mendeleyev went so far as to give a detailed description of physical or chemical properties of some elements which were as yet undiscovered. Time gave striking proof of his predictions and his periodic law. Mendeleyev published his conclusions in the first place by sending, early in March 186 9, a leaflet to many Russian and foreign scientists. It gave his system of elements based on their atomic weights and chemical resemblance. On the 18th March that year his paper on the subject was read at the meeting of the Russian Chemical Society, and two months later the Society's Journal published his article entitled "The correlation between properties of elements and their atomic weight". -

BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged To
BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged to Four and Five Significant Figures Norman E. Holden Energy Sciences & Technology Department National Nuclear Data Center Brookhaven National Laboratory P.O. Box 5000 Upton, NY 11973-5000 www.bnl.gov Prepared for the 44th IUPAC General Assembly, in Torino, Italy August 2007 Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CH10886 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. This preprint is intended for publication in a journal or proceedings. Since changes may be made before publication, it may not be cited or reproduced without the author’s permission. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Ment 98), Einsteinium (Element 99), Fermium (Element 100), Mendelevium (Element Downloaded by Guest on September 26, 2021 472 PHYSICS: G
VOL. 45, 1959 PHYSICS: G. T. SEABORG 471 of californium isotopes. The horizontal broken line labeled "0.02%" indicates the peak height for a group which would have that intensity relative to the total alpha particles. It is seen that the general background is considerably higher than this value, and, in fact, some of the peaks which are sketched in are hardly justifi- able in view of the scatter of the data. The bottom part of Figure 8 shows the same part of the spectrum taken under much improved conditions, as shown by the line illustrating the peak height of 0.02 per cent. All of the peaks are seen much more clearly and, in addition, one or two have showed up which were completely ob- scured under the other conditions. The principal change in technique which re- sulted in this improvement consisted of lining the interior of the spectrograph with very-low-atomic-number material which has the desirable effect of reducing low- angle scattering of alpha particles. In summary, we may say that during the past few years very important ad- vances in theory, as well as in experimental technique, are beginning to result in a systematic assignment of nuclear spectroscopic states and an interpretation of these states in terms of some detailed knowledge of nuclear structure. In this discussion, attention has been confined to one aspect of a very broad problem, namely, that having to do with spectroscopic states in the heavy-element region deduced from the alpha-decay process. There are many other experimental methods which are brought to bear on this problem, some of which have been touched upon in a peripheral sense in this discussion and others not mentioned at all. -

Americium-241 It''
It" • DOE/LLW-130 LP RECEIVE w. NOV 2 1 1995 O SCI $ National Low-Level Waste Management Program 8$ Radionuclide Report Series V-' Volume 14: Americium-241 it'' Nations/ Low-Level Waste Management Program 1 September 1995 ^ m ^^^^mm^^mm^-^n^ EriV DiSTfiiBUTiON OF WIS DOCUMENT IS UHLMTED DOE/LLW-130 National Low-Level Waste Management Program Radionuclide Report Series Volume 14: Americium-241 M. R. Winberg R. S. Garcia Published September 1995 Idaho National Engineering Laboratory Lockheed Idaho Technologies Company Idaho Falls, Idaho 83415 Prepared for the U.S. Department of Energy Assistant Secretary for Environmental Management Under DOE Idaho Operations Office Contract DE-AC07-94ID13223 DISTRIBUTION OF ISIS DOCUMENT 18 UNLBTED ° ' »* DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. ABSTRACT This report, Volume 14 of the National Low-Level Waste Management Program Radionuclide Report Series, discusses the radiological and chemical characteristics of americium-241 (241Am). This report also includes discussions about waste types and forms in which 241Am can be found and 241Am behavior in the environment and in the human body. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsi• bility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

Atomic Weights of the Elements 2011 (IUPAC Technical Report)*
Pure Appl. Chem., Vol. 85, No. 5, pp. 1047–1078, 2013. http://dx.doi.org/10.1351/PAC-REP-13-03-02 © 2013 IUPAC, Publication date (Web): 29 April 2013 Atomic weights of the elements 2011 (IUPAC Technical Report)* Michael E. Wieser1,‡, Norman Holden2, Tyler B. Coplen3, John K. Böhlke3, Michael Berglund4, Willi A. Brand5, Paul De Bièvre6, Manfred Gröning7, Robert D. Loss8, Juris Meija9, Takafumi Hirata10, Thomas Prohaska11, Ronny Schoenberg12, Glenda O’Connor13, Thomas Walczyk14, Shige Yoneda15, and Xiang-Kun Zhu16 1Department of Physics and Astronomy, University of Calgary, Calgary, Canada; 2Brookhaven National Laboratory, Upton, NY, USA; 3U.S. Geological Survey, Reston, VA, USA; 4Institute for Reference Materials and Measurements, Geel, Belgium; 5Max Planck Institute for Biogeochemistry, Jena, Germany; 6Independent Consultant on MiC, Belgium; 7International Atomic Energy Agency, Seibersdorf, Austria; 8Department of Applied Physics, Curtin University of Technology, Perth, Australia; 9National Research Council of Canada, Ottawa, Canada; 10Kyoto University, Kyoto, Japan; 11Department of Chemistry, University of Natural Resources and Applied Life Sciences, Vienna, Austria; 12Institute for Geosciences, University of Tübingen, Tübingen, Germany; 13New Brunswick Laboratory, Argonne, IL, USA; 14Department of Chemistry (Science) and Department of Biochemistry (Medicine), National University of Singapore (NUS), Singapore; 15National Museum of Nature and Science, Tokyo, Japan; 16Chinese Academy of Geological Sciences, Beijing, China Abstract: The biennial review of atomic-weight determinations and other cognate data has resulted in changes for the standard atomic weights of five elements. The atomic weight of bromine has changed from 79.904(1) to the interval [79.901, 79.907], germanium from 72.63(1) to 72.630(8), indium from 114.818(3) to 114.818(1), magnesium from 24.3050(6) to the interval [24.304, 24.307], and mercury from 200.59(2) to 200.592(3). -

10 CFR Ch. I (1–1–16 Edition)
Pt. 20, App. D 10 CFR Ch. I (1–1–16 Edition) Quantity Quantity Radionuclide (μCi) Radionuclide (μCi) Curium-242 .......................................................... 0 .01 Fermium-252 ....................................................... 1 Curium-243 .......................................................... 0 .001 Fermium-253 ....................................................... 1 Curium-244 .......................................................... 0 .001 Fermium-254 ....................................................... 10 Curium-245 .......................................................... 0 .001 Fermium-255 ....................................................... 1 Curium-246 .......................................................... 0 .001 Fermium-257 ....................................................... 0 .01 Curium-247 .......................................................... 0 .001 Mendelevium-257 ................................................ 10 Curium-248 .......................................................... 0 .001 Mendelevium-258 ................................................ 0.01 Curium-249 .......................................................... 1,000 Any radionuclide other than alpha emitting Berkelium-245 ...................................................... 100 radionuclides not listed above, or mixtures of Berkelium-246 ...................................................... 100 beta emitters of unknown composition ............ 0 .01 Berkelium-247 ..................................................... -

FERMIUM Element Symbol: Fm Atomic Number: 100
FERMIUM Element Symbol: Fm Atomic Number: 100 An initiative of IYC 2011 brought to you by the RACI CATH DE LITTLE www.raci.org.au FERMIUM Element symbol: Fm Atomic number: 100 Fermium, a synthetic element, is the 100th element in the periodic table and a member of the actinide series. It is the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities. A total of 19 isotopes are known, with257 Fm being the longest- lived one with a half-life of 100.5 days. Fermium was first discovered in the debris from the ‘Ivy Mike’ nuclear test on the 1st November 1952, this was the first successful test of a hydrogen bomb. The discovery of fermium (Z = 100) required more material, as the yield was expected to be at least an order of magnitude lower than that of element 99, and so contaminated coral from the Eniwetok atoll, where the test had taken place, was shipped to the University of California Radiation Laboratory in Berkeley, California, for processing and analysis. This discovery, and the new data on neutron capture, was initially kept secret on the orders of the U.S. military until 1955 due to Cold War tensions. Nevertheless, the Berkeley team was able to prepare elements 99 and 100 by civilian means, through the neutron bombardment of plutonium-239, and published this work in 1954 with the disclaimer that it was not the first studies that had been carried out on the elements. -

Tracking the Isotopes
explosion, These data would provide infor- mation on the change in amount of dense absorber between the source and the detec- tors and thus on the change in configuration of (he pit caused by the implosion, The key to this diagnostic scheme was to find such a What Are gamma ray source. The fission products barium-140 and lanthanum-140 (the same isotopes that led to the discovery, of fission) Radiochemistry and provided the solution. Barium-140 has a half-life of 12.8 days and decays to lantha- num 140. which has a 40-hour half life and Nuclear Chemistry? emits an energetic gamma ray with its decay. As the operation was finally worked out. the lanthanum-140 was extracted from a large he definition of the terms “radiochemistry” and “nuclear chemistry,” and batch of its barium 140 “parent” (obtained T the delineation of the presumed difference between them, is a problem that from Oak Ridge) and put into a compact bedevils the toilers in this vineyard whenever they try to tell what they do. receivcr in the center of the pit to be tested, Historically, radiochemistry appears to have been the first in line, When Chemical separation and further purification Madame Curie was carrying through her laborious chemical procedures to of the lanthanum-140 were carried out by a isolate and identify the radioactive elements and to establish their transforma- group headed by Gerhart Friedlander at a tions, she was doing radiochemistry. It was not until some time later that the tcmporary building a mile or two from the nature of the nucleus and its reactions became clear, In most of the early firing site in Bayo Canyon. -
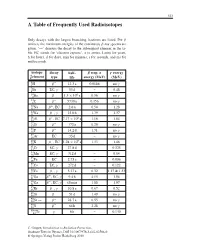
A Table of Frequently Used Radioisotopes
323 A Table of Frequently Used Radioisotopes Only decays with the largest branching fractions are listed. For β emitters the maximum energies of the continuous β-ray spectra are given. ‘→’ denotes the decay to the subsequent element in the ta- ble. EC stands for ‘electron capture’, a (= annus, Latin) for years, h for hours, d for days, min for minutes, s for seconds, and ms for milliseconds. isotope decay half- β resp. α γ energy A Z element type life energy (MeV) (MeV) 3 β− . γ 1H 12 3a 0.0186 no 7 γ 4Be EC, 53 d – 0.48 10 β− . × 6 γ 4Be 1 5 10 a 0.56 no 14 β− γ 6C 5730 a 0.156 no 22 β+ . 11Na ,EC 2 6a 0.54 1.28 24 β− γ . 11Na , 15 0h 1.39 1.37 26 β+ . × 5 13Al ,EC 7 17 10 a 1.16 1.84 32 β− γ 14Si 172 a 0.20 no 32 β− . γ 15P 14 2d 1.71 no 37 γ 18Ar EC 35 d – no 40 β− . × 9 19K ,EC 1 28 10 a 1.33 1.46 51 γ . 24Cr EC, 27 8d – 0.325 54 γ 25Mn EC, 312 d – 0.84 55 . 26Fe EC 2 73 a – 0.006 57 γ 27Co EC, 272 d – 0.122 60 β− γ . 27Co , 5 27 a 0.32 1.17 & 1.33 66 β+ γ . 31Ga , EC, 9 4h 4.15 1.04 68 β− γ 31Ga , EC, 68 min 1.88 1.07 85 β− γ .