Filipendula Ulmaria (L.) Maxim
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Habitat Management Plan Phoenix Park 2008
Phoenix Park Habitat Management Plan Report commissioned by The Office of Public Works Mary Tubridy and Associates August 2008 Clontarf, Dublin 3 Tel 01-8333195 Phoenix Park Habitat Management Plan Contents Acknowledgements 3 Summary 4 1 Methodology 1.1 Habitat map 5 1.2 Management plan 5 2 Habitats 2. 1 Introduction 6 2.2 Wetland habitats 8 2.2.1 Flora 8 2.2.2 Biodiversity evaluation 9 2. 3 Grasslands 9 2.3.1 Flora 10 2.3.2 Biodiversity evaluation 11 2.4 Woodlands 11 2.4.1 Flora 11 2.4.2 Biodiversity evaluation 12 3 Habitat Management Plan 3.1 Objectives 13 3.2 Short to medium term actions 13 3.2.1 Habitat and species diversity 13 3.2.2 Grassland management 14 3.2.3 Woodland management 14 3.2.4 Wetland management 15 3.2.5 Mammals and birds 15 3.2.6 Research, monitoring and education 16 References 17 Appendix 1 Notes on habitat diversity in Dublin 18 Mary Tubridy and Associates 2 Phoenix Park Habitat Management Plan Acknowledgements The study benefited from practical assistance, information and advice from the following organizations and individuals: OPW staff involved in the management of the Phoenix Park, particularly Dr John McCullen Margaret Gormley and Gabriel Gleeson. Managers of enclosures (Áras, United States Ambassador’s Residence, Zoo). Drs Linda Patton and Maurice Eakin of the National Parks and Wildlife Service The researchers who carried out specialist studies. They include Tom Hayden and his students from UCD (fallow deer, grey squirrel and mammals); Joe Caffrey and John Coyne of the Central Fisheries Board (freshwater habitats); Olivia Crowe and colleagues in BirdWatch Ireland (birds), Paul Scott of ScottCawley Ltd (bats) and Robbie Meehan (geodiversity). -

Plants in Cardiology
Double blind crossover comparison ofDDD and VVIR pacing in complete heart block 193 raised plasma atrial natriuretic peptide concentrations in 27 Pehrsson SK, Hjemdahl P, Nordlander R, Astrom H. A complete atrioventricular block. BMJ 1988;296:94. comparison of sympathoadrenal activity and cardiac 23 Follenius M, Brandenburger G. Increase in atrial natriuretic performance at rest and during exercise in patients with peptide in response to physical exercise. Eur J Appl ventricular demand or atrial synchronous pacing. Br Physiol 1988;57:159-62. Heart J 1988;60:212-20. 24 Svanegaard J, Angelo-Nielsen K, Hansen JS. Physiological 28 Kruse I, Amman K, Conradson T-B, et al. A comparison of hypertrophy of the heart and atrial natriuretic peptide acute and long-term hemodynamic effects of ventricular during rest and exercise. Br Heart J 1989;62:445-9. inhibited and atrial synchronous ventricular inhibited Br Heart J: first published as 10.1136/hrt.65.4.193 on 1 April 1991. Downloaded from 25 Ellenbogen KA, Kapadia K, Walsh M, Mohanty PK. pacing. Circulation 1982;65:846-55. Increase in plasma atrial natriuretic factor during 29 Rosenqvist M, Brandt J, Schuller H. Long-term pacing in ventriculoatrial pacing. Am J Cardiol 1989;64:236-7. sinus node disease: effects of stimulation mode on 26 Travill CM, Williams TDM, Vardas P, et al. Pacemaker cardiovascular morbidity and mortality. Am Heart J syndrome is associated with very high plasma concentra- 1988;116:16-22. tions ofatrial natriuretic peptide (ANF) [Abstract]. J Am 30 Sutton R, Kenny RA. The natural history of sick sinus Coll Cardiol 1989;13:111. -

FLORA from FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE of MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2
ISSN: 2601 – 6141, ISSN-L: 2601 – 6141 Acta Biologica Marisiensis 2018, 1(1): 60-70 ORIGINAL PAPER FLORA FROM FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE OF MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2 1Department of Pharmaceutical Botany, University of Medicine and Pharmacy of Tîrgu Mureş, Romania 2Mureş County Museum, Department of Natural Sciences, Tîrgu Mureş, Romania *Correspondence: Silvia OROIAN [email protected] Received: 2 July 2018; Accepted: 9 July 2018; Published: 15 July 2018 Abstract The aim of this study was to identify a potential source of medicinal plant from Transylvanian Plain. Also, the paper provides information about the hayfields floral richness, a great scientific value for Romania and Europe. The study of the flora was carried out in several stages: 2005-2008, 2013, 2017-2018. In the studied area, 397 taxa were identified, distributed in 82 families with therapeutic potential, represented by 164 medical taxa, 37 of them being in the European Pharmacopoeia 8.5. The study reveals that most plants contain: volatile oils (13.41%), tannins (12.19%), flavonoids (9.75%), mucilages (8.53%) etc. This plants can be used in the treatment of various human disorders: disorders of the digestive system, respiratory system, skin disorders, muscular and skeletal systems, genitourinary system, in gynaecological disorders, cardiovascular, and central nervous sistem disorders. In the study plants protected by law at European and national level were identified: Echium maculatum, Cephalaria radiata, Crambe tataria, Narcissus poeticus ssp. radiiflorus, Salvia nutans, Iris aphylla, Orchis morio, Orchis tridentata, Adonis vernalis, Dictamnus albus, Hammarbya paludosa etc. Keywords: Fărăgău, medicinal plants, human disease, Mureş County 1. -

Introduction to Neotropical Entomology and Phytopathology - A
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol. VI - Introduction to Neotropical Entomology and Phytopathology - A. Bonet and G. Carrión INTRODUCTION TO NEOTROPICAL ENTOMOLOGY AND PHYTOPATHOLOGY A. Bonet Department of Entomology, Instituto de Ecología A.C., Mexico G. Carrión Department of Biodiversity and Systematic, Instituto de Ecología A.C., Mexico Keywords: Biodiversity loss, biological control, evolution, hotspot regions, insect biodiversity, insect pests, multitrophic interactions, parasite-host relationship, pathogens, pollination, rust fungi Contents 1. Introduction 2. History 2.1. Phytopathology 2.1.1. Evolution of the Parasite-Host Relationship 2.1.2. The Evolution of Phytopathogenic Fungi and Their Host Plants 2.1.3. Flor’s Gene-For-Gene Theory 2.1.4. Pathogenetic Mechanisms in Plant Parasitic Fungi and Hyperparasites 2.2. Entomology 2.2.1. Entomology in Asia and the Middle East 2.2.2. Entomology in Ancient Greece and Rome 2.2.3. New World Prehispanic Cultures 3. Insect evolution 4. Biodiversity 4.1. Biodiversity Loss and Insect Conservation 5. Ecosystem services and the use of biodiversity 5.1. Pollination in Tropical Ecosystems 5.2. Biological Control of Fungi and Insects 6. The future of Entomology and phytopathology 7. Entomology and phytopathology section’s content 8. ConclusionUNESCO – EOLSS Acknowledgements Glossary Bibliography Biographical SketchesSAMPLE CHAPTERS Summary Insects are among the most abundant and diverse organisms in terrestrial ecosystems, making up more than half of the earth’s biodiversity. To date, 1.5 million species of organisms have been recorded, although around 85% of potential species (some 10 million) have not yet been identified. In the case of the Neotropics, although insects are clearly a vital element, there are many families of organisms and regions that are yet to be well researched. -

Meadowsweet 2015
ONLINE SERIES MONOGRAPHS The Scientific Foundation for Herbal Medicinal Products Filipendulae ulmariae herba Meadowsweet 2015 www.escop.com The Scientific Foundation for Herbal Medicinal Products FILIPENDULAE ULMARIAE HERBA Black Cohosh 2015 ESCOP Monographs were first published in loose-leaf form progressively from 1996 to 1999 as Fascicules 1-6, each of 10 monographs © ESCOP 1996, 1997, 1999 Second Edition, completely revised and expanded © ESCOP 2003 Second Edition, Supplement 2009 © ESCOP 2009 ONLINE SERIES ISBN 978-1-901964-37-0 Filipendulae ulmariae herba - Meadowsweet © ESCOP 2015 Published by the European Scientific Cooperative on Phytotherapy (ESCOP) Notaries House, Chapel Street, Exeter EX1 1EZ, United Kingdom www.escop.com All rights reserved Except for the purposes of private study, research, criticism or review no part of this text may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, without the written permission of the publisher. Important Note: Medical knowledge is ever-changing. As new research and clinical experience broaden our knowledge, changes in treatment may be required. In their efforts to provide information on the efficacy and safety of herbal drugs and herbal preparations, presented as a substantial overview together with summaries of relevant data, the authors of the material herein have consulted comprehensive sources believed to be reliable. However, in view of the possibility of human error by the authors or publisher of the work herein, or changes in medical knowledge, neither the authors nor the publisher, nor any other party involved in the preparation of this work, warrants that the information contained herein is in every respect accurate or complete, and they are not responsible for any errors or omissions or for results obtained by the use of such information. -
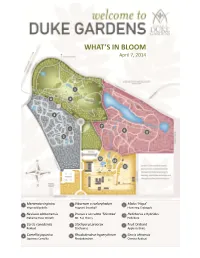
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Top 6 Herbs to Heal Pain and Inflammation in Horses
Top 6 Herbs to Heal Pain and Inflammation in Horses Discover the Top Herbs to Manage your Horse’s Pain and Inflammation - Naturally Why Herbal Medicine? It is understood that animals have been self-medicating with herbs throughout time. In fact, the same can be said for humans. Did you know that many pharmaceutical drugs have been developed as a result of studying traditional herbal medicines, extracting what are considered to be the key chemical constituents, synthetically manufacturing those chemicals and prescribing them at high doses? In many developing countries, a large portion of the population still rely heavily on traditional practitioners and medicinal plants to meet their primary health care needs, even where more modern Western medical systems are available. So why use herbal medicines when there are pharmaceutical alternatives? What we are unable to replicate in pharmaceutical drugs is the manner in which the multitude of different phytochemicals found in plant medicines work together synergistically to heal the body. Taking a single chemical component from a plant and prescribing it at a high dose often produces side effects that are not evident when the medicine is taken in plant form, as plants tend to contain just the right balance of a range of phytochemicals. This is not to say that plant medicines have no side effects, as some are toxic when prescribed incorrectly. Another factor to consider is cost. Herbal medicines are often significantly cheaper than pharmaceutical medicines, largely because herbal medicines cannot be patented. In some cases, you can even grow fresh herbs in your own home. -

Purple Loosestrife May Impede Boat Travel
Pickeral Weed: Fireweed: Why Should Purple Pontederia cordata Epilobium angustifolium Loosestrife Concern Flowers 2-lipped, spikes Fatter spikes of 4-petaled, 3”-4”; leaves heart shaped, stalked flowers; alternate, You? single; water, 1’ to 3’ toothed leaves; northern plant of drier areas; 2’ to 6’ False Dragonhead: Plant diversity in wetlands Physostegia virginiana ✤ Tubular flowers, dissimilar petals; declines dramatically and toothed leaves; 1’ to 5’ (Other large mint many rare and endangered family plants: Hedge Nettle, Giant Hyssop) plants found in our remaining Swamp Loosestrife: Smartweed: wetlands are threatened. Decodon verticillatus Polygonum sp. Flowers bunched at (many native well-separated leaf species) - Tiny Most wetland animals that Look-a-likes ✤ bases; leaves whorled flowers, skinny depend on native plants for in 3s or 4s; stems spikes 1” to 4”; food and shelter decline usually arching, 1’ to 8’ alternate leaves clasp stem at base; significantly. Some species, stems jointed, 1’ to 6’ such as Baltimore butterflies, marsh wrens, and least bitterns may disappear entirely. Lupine: Blue Vervain: Lupinus perennis Verbena hastata Pea-like flowers; alternate, PURPLE (+ other Verbena sp.) ✤ Recreational uses of wetlands palm-like leaves; dry, LOOSESTRIFE Flowers tiny, pencil thin for hunting, trapping, fishing, sandy places; 2’ to 4’ spikes; toothed, oval, stalked leaves; moist bird watching and nature study to dry places; 2’ to 6’ decrease. Thick growth of purple loosestrife may impede boat travel. Winged Loosestrife: Steeplebush: ✤ Wetlands may store and filter Lythrum alatum Spiraea tomentosa less water. Smaller, single flowers DO NOT CONFUSE THESE Tiny flowers, conical at well-separated leaf set of flower spikes; bases; upper leaves NATIVE SPECIES WITH alternate, oval ✤ Millions of dollars spent to single; southern PURPLE LOOSESTRIFE! leaves; woody stem preserve wetlands would be prairies, 2’ to 3’ 1’ to 4’ wasted. -

Indiana Medical History Museum Guide to the Medicinal Plant Garden
Indiana Medical History Museum Guide to the Medicinal Plant Garden Garden created and maintained by Purdue Master Gardeners of Marion County IMHM Medicinal Plant Garden Plant List – Common Names Trees and Shrubs: Arborvitae, Thuja occidentalis Culver’s root, Veronicastrum virginicum Black haw, Viburnum prunifolium Day lily, Hemerocallis species Catalpa, Catalpa bignonioides Dill, Anethum graveolens Chaste tree, Vitex agnus-castus Elderberry, Sambucus nigra Dogwood, Cornus florida Elecampane, Inula helenium Elderberry, Sambucus nigra European meadowsweet, Queen of the meadow, Ginkgo, Ginkgo biloba Filipendula ulmaria Hawthorn, Crateagus oxycantha Evening primrose, Oenothera biennis Juniper, Juniperus communis False Solomon’s seal, Smilacina racemosa Redbud, Cercis canadensis Fennel, Foeniculum vulgare Sassafras, Sassafras albidum Feverfew, Tanacetum parthenium Spicebush, Lindera benzoin Flax, Linum usitatissimum Witch hazel, Hamamelis virginiana Foxglove, Digitalis species Garlic, Allium sativum Climbing Vines: Golden ragwort, Senecio aureus Grape, Vitis vinifera Goldenrod, Solidago species Hops, Humulus lupulus Horehound, Marrubium vulgare Passion flower, Maypop, Passiflora incarnata Hyssop, Hyssopus officinalis Wild yam, Dioscorea villosa Joe Pye weed, Eupatorium purpureum Ladybells, Adenophora species Herbaceous Plants: Lady’s mantle, Alchemilla vulgaris Alfalfa, Medicago sativa Lavender, Lavendula angustifolia Aloe vera, Aloe barbadensis Lemon balm, Melissa officinalis American skullcap, Scutellaria laterifolia Licorice, Glycyrrhiza -

Anti-Urolithiatic Activity of Filipendula Ulmaria Leaf Extracts in Male Albino Rats
CATRINA (2020), 22(1): 49-55 © 2020 BY THE EGYPTIAN SOCIETY FOR ENVIRONMENTAL SCIENCES Anti-Urolithiatic Activity of Filipendula ulmaria Leaf Extracts in Male Albino Rats Rabab M. Aljarari*1 and Muna O. Alamoudi2 1 Biology Department, Faculty of Science, Jeddah University, Jeddah - Saudi Arabia 2 Biology Departments, Faculty of Science, Hail University, Hail City - Saudi Arabia ABSTRACT Filipendula ulmaria L. is a perennial herb that can be found in regions with higher humidity in Asia, and Europe. The herb is used medicinally as drugs for several purposes such as facilitating renal elimination functions and many other biological activities. Therefore, the present study was focus on the anti-Urolithiatic activity of Filipendula ulmaria leaf extracts using Albino rats as a model. The influence of oral administration of aqueous, methanolic and ethanolic extracts of Filipendula ulmaria leaves on accumulated calcium oxalate urolithiasis has been investigated. Nephrolithiasis was induced in the rats by oral administration of ethylene glycol (0.75%) in drinking water for 28 days. Animals were divided into nine groups, each containing six rats. Group 1 received purified water as negative control; group 2 received ethylene glycol in drinking water; group 3 received cystone as curative agent, groups 4-9 received Filipendula ulmaria extracts in dosages of 100 and 200 mg/kg body weight, respectively. The aqueous and methanolic Leaf-extracts showed significant reduction in the urine parameters compared to ethanolic extracts. However, using the cystone showed highest reduction rate compared to the leaf-extracts using different solvents. Cystone also recorded higher impact on the serum parameters compared to the extract used. -

Site Synopsis
SITE SYNOPSIS SITE NAME : LOWER RIVER SUIR SITE CODE : 002137 This site consists of the freshwater stretches of the River Suir immediately south of Thurles, the tidal stretches as far as the confluence with the Barrow/Nore immediately east of Cheekpoint in Co. Waterford and many tributaries including the Clodiagh in Co. Waterford, the Lingaun, Anner, Nier, Tar, Aherlow, Multeen and Clodiagh in Co. Tipperary. The Suir and its tributaries flows through the counties of Tipperary, Kilkenny and Waterford. Upstream of Waterford city, the swinging meanders of the Suir criss- cross the Devonian sandstone rim of hard rocks no less than three times as they leave the limestone-floored downfold below Carrick In the vicinity of Carrick-on-Suir the river follows the limestone floor of the Carrick Syncline. Upstream of Clonmel the river and its tributaries traverse Upper Palaeozoic Rocks, mainly the Lower Carboniferous Visean and Tournaisian. The freshwater stretches of the Clodiagh River in Co. Waterford traverse Silurian rocks, through narrow bands of Old Red Sandstone and Lower Avonian Shales before reaching the carboniferous limestone close to its confluence with the Suir. The Aherlow River flows through a Carboniferous limestone valley, with outcrops of Old Red Sandstone forming the Galtee Mountains to the south and the Slievenamuck range to the north. Glacial deposits of sands and gravels are common along the valley bottom, flanking the present-day river course. The site is a candidate SAC selected for the presence of the priority habitats on Annex I of the E.U. Habitats Directive - alluvial wet woodlands and Yew Wood. -

Dorset Moths (Vc9) Annual Report 2019
DORSET MOTHS (VC9) ANNUAL REPORT 2019 Paul Butter, Phil Sterling, Mike Hetherington, Jack Oughton & Alison Stewart 1 CONTENTS Introduction Mike Hetherington 2 Highlights of the Year Jack Oughton 4 Summary of 2019 Records Alison Stewart 6 List of Recorders 8 Macro Moths 2019 Paul Butter & Mike Hetherington 9 Micro Moths 2019 Phil Sterling 27 Migrant Moth Report 2019 Paul Butter & Jack Oughton 36 Dearth of Daytime Observations Paul Butter 39 Dorset Moths Annual Meeting 2019 Mike Hetherington 40 Grass Webworms in Dorset 2019 Mike Hetherington 41 The Geometrician Grammodes stolida – a first for Dorset, recorded day-flying on Portland on 24/09. Photo of that record © Bob Johnson. Moitrelia obductella – another Dorset first for the year, found as larvae on Marjoram Origanum vulgare Wyke Regis on 20/06. Photo of adult raised from larva by Dave Foot © Paul Harris. Front cover images © Mike Hetherington (Cream-spot Tiger & Elephant Hawk-moth), Paul Butter (Forester), Paul Harris (Ancylolomia tentaculella). DMG Logo © Chris Manley 2 INTRODUCTION Welcome to the Dorset Moths Annual Report for 2019. As many of you will be aware, a new verification team took over when Les Evans-Hill stepped down as County Moth Recorder at the end of 2016. The current team members are: Adrian Bicker (Living Record), Terry Box, Paul Butter, Pete Forrest, Julian Francis, Mike Hetherington, Tom Morris, Jack Oughton, Phil Sterling (micro moth County Moth Recorder) and Alison Stewart (Dorset Environmental Records Centre). After addressing a backlog in the verification of records for 2017 and 2018 the team is now in a position to produce an Annual Report for 2019.