Cucurbit Seed Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Music Initiative Jka Peer - Reviewed Journal of Music
VOL. 01 NO. 01 APRIL 2018 MUSIC INITIATIVE JKA PEER - REVIEWED JOURNAL OF MUSIC PUBLISHED,PRINTED & OWNED BY HIGHER EDUCATION DEPARTMENT, J&K CIVIL SECRETARIAT, JAMMU/SRINAGAR,J&K CONTACT NO.S: 01912542880,01942506062 www.jkhighereducation.nic.in EDITOR DR. ASGAR HASSAN SAMOON (IAS) PRINCIPAL SECRETARY HIGHER EDUCATION GOVT. OF JAMMU & KASHMIR YOOR HIGHER EDUCATION,J&K NOT FOR SALE COVER DESIGN: NAUSHAD H GA JK MUSIC INITIATIVE A PEER - REVIEWED JOURNAL OF MUSIC INSTRUCTION TO CONTRIBUTORS A soft copy of the manuscript should be submitted to the Editor of the journal in Microsoft Word le format. All the manuscripts will be blindly reviewed and published after referee's comments and nally after Editor's acceptance. To avoid delay in publication process, the papers will not be sent back to the corresponding author for proof reading. It is therefore the responsibility of the authors to send good quality papers in strict compliance with the journal guidelines. JK Music Initiative is a quarterly publication of MANUSCRIPT GUIDELINES Higher Education Department, Authors preparing submissions are asked to read and follow these guidelines strictly: Govt. of Jammu and Kashmir (JKHED). Length All manuscripts published herein represent Research papers should be between 3000- 6000 words long including notes, bibliography and captions to the opinion of the authors and do not reect the ofcial policy illustrations. Manuscripts must be typed in double space throughout including abstract, text, references, tables, and gures. of JKHED or institution with which the authors are afliated unless this is clearly specied. Individual authors Format are responsible for the originality and genuineness of the work Documents should be produced in MS Word, using a single font for text and headings, left hand justication only and no embedded formatting of capitals, spacing etc. -

The Heirloom Gardener's Seed-Saving Primer Seed Saving Is Fun and Interesting
The Heirloom Gardener's Seed-Saving Primer Seed saving is fun and interesting. It tells the story of human survival, creativity, and community life. Once you learn the basics of saving seeds you can even breed your own variety of crop! Share your interesting seeds and stories with other gardeners and farmers while helping to prevent heirloom varieties from going extinct forever. Contact The Foodshed Project to find out about local seed saving events! 1. Food “as a system”...........................................................................................................................5 2. Why are heirloom seeds important?.................................................................................................6 3. How are plants grouped and named?...............................................................................................8 4. Why is pollination important?... ......................................................................................................11 5. What is a monoecious or a dioecious plant?....................................................................................12 6. How do you know if a plant will cross-breed?.................................................................................14 7. What types of seeds are easiest to save?........................................................................................18 8. What about harvesting and storing seeds?.....................................................................................20 9. What do I need to know -

Basil Dolce Fresca
BASIL DOLCE FRESCA Ocimum basilicum BASIL PERSIAN Ocimum basilicum BEAN MASCOTTE Phaseolus vulgaris BEAN SEYCHELLES Phaseolus vulgaris BEET AVALANCHE Beta vulgaris BROCCOLI ARTWORK F1 Brassica oleracea Italica BRUSSELS SPROUTS HESTIA F1 Brassica oleracea CABBAGE KATARINA F1 Brassica oleracea (Capitata group) CHIVES, GARLIC GEISHA Allium tuberosum CUCUMBER PARISIAN GHERKIN F1 Cucumis sativus CUCUMBER PICK A BUSHEL F1 Cucumis sativus CUCUMBER SALADMORE BUSH F1 Cucumis sativus EGGPLANT PATIO BABY F1 Solanum melongena FENNEL ANTARES F1 Foeniculum vulgare KALE PRIZM F1 Brassica oleracea (Acephala Group) KOHLRABI KONAN F1 Brassica oleracea LETTUCE SANDY Lactuca sativa MELON MELEMON F1 Cucumis melo L. MIZUNA RED KINGDOM F1 Brassica juncea OKRA CANDLE FIRE F1 Abelmoschus esculentus ONION, BUNCHING WARRIOR Allium fistulosusm OREGANO CLEOPATRA Origanum syriaca PAK CHOI BOPAK F1 Brassica rapa chinensis PEA PATIO PRIDE Pisum sativum PEPPER AJI RICO F1 Capsicum baccatum PEPPER CHILI PIE F1 Capsicum annuum PEPPER CORNITO GIALLO F1 Capsicum annuum PEPPER EMERALD FIRE F1 Capsicum annuum PEPPER ESCAMILLO F1 Capsicum annuum PEPPER FLAMING FLARE F1 Capsicum annuum PEPPER GIANT RISTRA F1 Capsicum annuum PEPPER HOT SUNSET F1 Capsicum annuum PEPPER MAD HATTER F1 Capsicum baccatum PEPPER MAMA MIA GIALLO F1 Capsicum annuum PEPPER PRETTY N SWEET F1 Capsicum annuum PEPPER SWEET SUNSET F1 Capsicum annuum PEPPER SWEETIE PIE F1 Capsicum annuum PEPPER SERRANO FLAMING JADE F1 Capsicum annuum PUMPKIN CINDERELLA'S CARRIAGE F1 Cucurbita maxima PUMPKIN PEPITAS F1 Cucurbita pepo PUMPKIN SUPER MOON F1 Cucurbita maxima RADISH RIVOLI Raphanus sativus RADISH ROXANNE F1 Raphanus sativus RADISH SWEET BABY F1 Raphanus sativus SQUASH BOSSA NOVA F1 Cucurbita pepo SQUASH BUTTERSCOTCH F1 Cucurbita moschata SQUASH HONEYBABY F1 Cucurbita moschata SQUASH SUGARETTI F1 Cucurbita pepo STRAWBERRY DELIZZ F1 Fragaria F. -

Effects of Cucumis Metuliferus (Cucurbitaceae) Fruits on Enzymes and Haematological Parameters in Albino Rats
African Journal of Biotechnology Vol. 6 (22) pp. 2515-2518, 19 November, 2007 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2007 Academic Journals Full Length Research Paper Effects of Cucumis metuliferus (Cucurbitaceae) fruits on enzymes and haematological parameters in albino rats Noel N. Wannang*, Nanloh S. Jimam, Simeon Omale, Maxwell L. P. Dapar, Steven S. Gyang and John C. Aguiyi Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmaceutical Sciences, University of Jos, Nigeria. Accepted 24 October, 2007 The effects of the powdered fruits of Cucumis metuliferus on enzymes and haematological indices were evaluated in adult albino rats. The study revealed a significant (P<0.05) dose-dependent decrease in white blood cells (WBC) count. 500 mg/kg body weight of the powdered fruit produced a significant (P<0.05) decrease in red blood cells (RBC), and an increase in platelet and haemoglobin (Hb), while there was an insignificant (P>0.05) decrease in clotting and bleeding time. 1000 mg/kg produced significant (P<0.05) increase in RBC, platelets, Hb and packed cell volume (PCV) values, and an insignificant (P>0.05) decrease in clotting and bleeding time. The biochemical parameters evaluation showed that 500 - 1000 mg/kg of the powdered fruit of the plant produced a dose-dependent significant (P<0.05) increase in the levels of serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), Blood urea nitrogen (BUN) and Total protein. This result showed that Cucumis metuliferus produced alterations in the haematological and biochemical indices evaluated. Keywords: Cucumis metuliferus, serum, enzymes, haematological. -

Watermelon Seed Oil: Its Extraction, Analytical Studies, Modification and Utilization in Cosmetic Industries
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 07 Issue: 02 | Feb 2020 www.irjet.net p-ISSN: 2395-0072 Watermelon Seed Oil: Its Extraction, Analytical studies, Modification and Utilization in Cosmetic Industries Sarfaraz Athar1, Abullais Ghazi2, Osh Chourasiya3, Dr. Vijay Y. Karadbhajne4 1,2,3Department of Oil Technology, Laxminarayan Institute of Technology, Nagpur 4Head, Dept. of Oil Technology, Professor, Laxminarayan Institute of Technology, Nagpur, Maharashtra, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - Watermelon seed is one of the unexplored seed in acid or omega 6 fatty acid (about 45-73%). Oleic, palmitic the world which is often discarded after eating the fruit. and stearic acid are also present in small quantities [3]. Researches show that these seeds contain nutrients like protein, essential fatty acids, vitamins and minerals. Oil Various researches report the positive effect of watermelon content in the seeds is between 35-40% and the unsaturated seed oil over skin. The oil is light, consists of humectants and fatty acid content in oil is 78-86% predominantly linoleic acid moisturising properties. It is easily absorbed by skin and (45-73%). This oil is effective for skin care as it is light, easily helps in restoring the elasticity of skin. Due to these absorbable and has humectants properties. Our study is about attributes this oil can be used in cosmetic industry for extraction of watermelon seed oil by solvent extraction process production of skin care products. The watermelon seed oil with the use of different solvents, its analysis and application can also be used as an anti inflammatory agent [4]. -

Origin of Plant Originated from Some Had Possibly Changed Recognise Original Wild Species. Suggested a Relationship with Pr
The possible origin of Cucumis anguria L. by A.D.J. Meeuse (National Herbarium, Pretoria) (Issued Oct. 2nd, 1958) Cucumis India Gherkin” “Bur anguria L., the “West or Gherkin”, is a cultigen known to have occurred in the West Indies in a cultivated or more or less adventitious state since before 1650 when the first accounts of this plant were published (1, 2). The occurrence of a single species of this old world genus — which is mainly African but extends through South West Asia to India — in America, combined with the fact that it is almost exclusively found in cultivation or as an escape, makes one feel suspicious about its being truly indigenous in the New World. Naudin (4) discussed the history of this plant and suggested that it introduced from West Africa whence slaves were was originally negro brought to the New World. However, he admittedly did not know any wild African species of Cucumis which resembles C. anguria sufficiently to deserve consideration as its probable ancestor. J. D. Hooker (5) also discussed the origin of the West Indian plant and was inclined to agree that it originated from some wild African ancestral form which had possibly changed so much through cultivation that it would be somewhat difficult to recognise the original wild species. He suggested a relationship with Cucumis prophetarum and “C. figarei” but he cautiously stated that these two species are perennial, whereas C. anguria is a typical annual. A. de Candolle (6) pointed out that although C. anguria was generally assumed to be a native of the Antilles, more particularly of Jamaica, two facts plead against this idea. -

Refining of the Egusi Locus in Watermelon Using KASP Assays
Scientia Horticulturae 257 (2019) 108665 Contents lists available at ScienceDirect Scientia Horticulturae journal homepage: www.elsevier.com/locate/scihorti Refining of the egusi locus in watermelon using KASP assays T ⁎ Lucky Paudela, Josh Clevengerb, Cecilia McGregorc, a Institute for Plant Breeding, Genetics & Genomics, University of Georgia, 1111 Plant Sciences Bldg., Athens, GA, 30602, USA b Institute for Plant Breeding, Genetics & Genomics, University of Georgia, 111 Riverbend Rd. CAGT, Athens, GA, 30602, USA c Department of Horticulture and Institute for Plant Breeding, Genetics & Genomics, University of Georgia, 1111 Plant Sciences Bldg., Athens, GA, 30602, USA ARTICLE INFO ABSTRACT Keywords: Egusi watermelon (Citrullus mucosospermus), a close relative of sweet watermelon, is an economically important Citrullus lanatus crop grown in many West African countries for its protein and lipid rich edible seeds. Egusi watermelon seeds Citrullus mucosospermus have a thick, fleshy mucilaginous seed coat layer surrounding the seed coat which is unique to egusi watermelon. Egusi watermelon The egusi seed trait is controlled by a single recessive Mendelian locus, eg, located on chromosome 6 from Seed coat type 6.75 Mb to 11.03 Mb. This region is 4.28 Mb wide and contains 241 candidate genes. The region lacks adequate QTL-seq markers for fine mapping and for marker-assisted selection (MAS) of the egusi trait. In this study, we used QTL- KASP assays seq to validate the position of the eg locus and to identify SNP markers to refine the locus. A genomic region associated with the egusi trait was confirmed on chromosome 6 from 5.25 Mb to 7.85 Mb partially overlapping the previously mapped eg locus. -

Mature Fruit Vegetables
27 Mature Fruit Vegetables MIKAL E. SALTVEIT University of California, Davis, Davis, California, U.S.A. I. INTRODUCTION Many vegetables are classified botanically as fruit, that is, as the product of a ripening ovary and its associated tissue. Fruit vegetables are consumed when they are immature or mature. This distinction is useful because each division has similar postharvest behavior and storage requirements (Table 1). Examples of immature fruit vegetables include cucum bers {Cucumis sativus L.), summer squash (Cucurbita pepo L.), and sweetcorn (Zea mays L. var. rugosa Bonaf.), while examples of mature fruit vegetables are chili peppers {Capsi cum annum L. var. annum Longum Group), melons {Cucumis melo L.), pumpkins {Cucur bita pepo L. and C. maxima Duchesne ex Lam.), tomatoes {Lycopersicon esculentum Mill.), watermelons [Citrullus lanatus (Thunb.) Matsum. & Nak.], and winter squash {Cu curbita maxima L.). These mature fruit vegetables are derived from a taxonomically di verse number of families, but the major mature fruit vegetables are dominated by species from the Cucurbitaceae (melons, pumpkins, and winter squash), and Solanaceae (peppers and tomatoes). (See Table 2.) Mature fruit vegetables can be berries (peppers, tomatoes) and pepos (cucurbits) (Rubatzky and Yamaguchi, 1997). Melons comprise a diverse group of fruits, with the two major groups being those that have a netted surface (Reticulatus group: cantaloupe, muskmelon) and those that are smooth (Inodorus group: honeydew, winter melons). Most fruit vegetables are warm-season crops that are subject to chilling injury (CI). (See Chap. 19.) Exceptions include sweetcorn and such cool-season crops as peas {Pisum sativum L.), broad beans {Viciafaba L.), and dried chili peppers. -

Iran Has the Second Largest Economy (After Saudi Arabia) and Population (After Egypt)
COUNTRY FACT SHEET ON FOOD AND AGRICULTURE POLICY TRENDS | September 2014 Socio-economic context and role of agriculture In the Near East and North Africa region, Iran has the second largest economy (after Saudi Arabia) and population (after Egypt). Iran ranks second in the world in natural gas reserves and third in oil reserves.1 The agriculture and rural sector share in the GDP has declined in the last twenty years and yet is the source of income for more than 15 million people in rural areas.2 One quarter of the rural population is landless and of those who own land, one third are smallholders. Those within this segment of the population often fall below or just within the poverty line and face high underemployment rates. IRAN Some of the main development challenges at the national level are the harsh conditions of the physical environment and low productivity of small-holder farmers. Food security challenges include lack of self-sufficiency in major staple crops and inadequate access to food in terms of quantity of daily energy intake. The Iranian Government has adopted a comprehensive strategy envisioning market-based reforms as reflected in the 20-year Vision document and Iran’s fifth Five-Year Development Plan (FYDP 2011–15). The Government envisioned a large privatization programme in its 2010-2015 five-year plan, aiming to privatize 20 percent of state-owned enterprises (SOEs) each year. Moreover, Iran’s 2012 Doing Business ranking is in the bottom tiers of the Middle East and North Africa (MENA) region, at 144th overall. Only Algeria, Iraq, and Djibouti rank lower among MENA countries. -
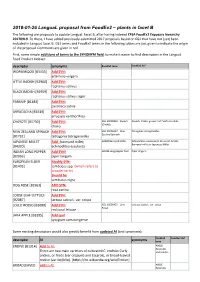
2018-01-26 Langual Proposal from Foodex2 – Plants in Facet B
2018-01-26 LanguaL proposal from FoodEx2 – plants in facet B The following are proposals to update LanguaL Facet B, after having indexed EFSA FoodEx2 Exposure hierarchy 20170919. To these, I have added previously-submitted 2017 proposals based on GS1 that have not (yet) been included in LanguaL facet B. GS1 terms and FoodEx2 terms in the following tables are just given to indicate the origin of the proposal. Comments are given in red. First, some simple additions of terms to the SYNONYM field, to make it easier to find descriptors in the LanguaL Food Product Indexer: descriptor synonyms FoodEx2 term FoodEx2 def WORMWOOD [B3433] Add SYN: artemisia vulgaris LITTLE RADISH [B2960] Add SYN: raphanus sativus BLACK RADISH [B2959] Add SYN: raphanus sativus niger PARSNIP [B1483] Add SYN: pastinaca sativa ARRACACHA [B3439] Add SYN: arracacia xanthorrhiza CHAYOTE [B1730] Add SYN: GS1 10006356 - Squash Squash, Choko, grown from Sechium edule (Choko) choko NEW ZEALAND SPINACH Add SYN: GS1 10006427 - New- Tetragonia tetragonoides Zealand Spinach [B1732] tetragonia tetragonoides JAPANESE MILLET Add : barnyard millet; A000Z Barnyard millet Echinochloa esculenta (A. Braun) H. Scholz, Barnyard millet or Japanese Millet. [B4320] echinochloa esculenta INDIAN LONG PEPPER Add SYN! A019B Long pepper fruit Piper longum [B2956] piper longum EUROPEAN ELDER Modify SYN: [B1403] sambucus spp. (which refers to broader term) Should be sambucus nigra DOG ROSE [B2961] ADD SYN: rosa canina LOOSE LEAF LETTUCE Add SYN: [B2087] lactusa sativa L. var. crispa LOLLO ROSSO [B2088] Add SYN: GS1 10006425 - Lollo Lactuca sativa L. var. crispa Rosso red coral lettuce JAVA APPLE [B3395] Add syn! syzygium samarangense Some existing descriptors would also greatly benefit from updated AI (and synonyms): FoodEx2 FoodEx2 def descriptor AI synonyms term ENDIVE [B1314] Add to AI: A00LD Escaroles There are two main varieties of cultivated C. -

Buffalo Gourd (Family Cucurbitaceae, Cucurbita Foetidissima)
Buffalo Gourd (Family Cucurbitaceae, Cucurbita foetidissima) By Gerald R Noonan PhD May 2013 © May 2013 Buffalo Gourds are a common sight along the trails near the San Pedro House and in many other parts of SPRNCA. They grow as a prostate vine that spreads along the ground and may grow up to 20 feet long. The leaves are relatively large, up to approximately a foot in length, grayish green above and whitish beneath. The triangular shaped leaves have fine teeth along the margins and are born on relatively long stalks. During approximately May to August, yellow flowers appear. They are funnel shaped, five-lobed, about 4 inches long, and have the basic ribbed and with veins. The flowers open very early in the day and are pollinated by bees. Pollinated flowers each produce a gourd that is approximately 4 inches long, round, and predominantly dark green but with light stripes. The gourds eventually mature to an even yellow color and with continued exposure to the sun become whitish in appearance. Buffalo Gourds are perennial, die back in the winter, and then grow back from the large root when weather becomes warmer. The triangular shaped leaves distinguish this plant from the other two gourd producing species of vines that occur in SPRNCA. Finger-leaved Gourds differ by having central silvery white markings on the tops of the five narrow fingerlike segments of each leaf. Melon Loco plants differ by having leaves that are roundish or kidney shaped, approximately 2-6 inches wide, and with irregular jagged edges or pleats. Buffalo Gourds occur in roadsides and in dry or sandy areas. -

Value Addition of Southern African Monkey Orange (Strychnos Spp.): Composition, Utilization and Quality Ruth Tambudzai Ngadze
Value addition of Southern African monkey orange ( Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Strychnos spp.): composition, utilization and quality Ruth Tambudzai Ngadze 2018 Ruth Tambudzai Ngadze Propositions 1. Food nutrition security can be improved by making use of indigenous fruits that are presently wasted, such as monkey orange. (this thesis) 2. Bioaccessibility of micronutrients in maize-based staple foods increases by complementation with Strychnos cocculoides. (this thesis) 3. The conclusion from Baker and Oswald (2010) that social media improve connections, neglects the fact that it concomitantly promotes solitude. (Journal of Social and Personal Relationships 27:7, 873–889) 4. Sustainable agriculture in developed countries can be achieved by mimicking third world small-holder agrarian systems. 5. Like first time parenting, there is no real set of instructions to prepare for the PhD journey. 6. Undertaking a sandwich PhD is like participating in a survival reality show. Propositions belonging to the thesis, entitled: Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Ruth T. Ngadze Wageningen, October 10, 2018 Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Ruth Tambudzai Ngadze i Thesis committee Promotor Prof. Dr V. Fogliano Professor of Food Quality and Design Wageningen University & Research Co-promotors Dr A. R. Linnemann Assistant professor, Food Quality and Design Wageningen University & Research Dr R. Verkerk Associate professor, Food Quality and Design Wageningen University & Research Other members Prof. M. Arlorio, Università degli Studi del Piemonte Orientale A. Avogadro, Italy Dr A. Melse-Boonstra, Wageningen University & Research Prof.