Ichthyological Exploration of Freshwaters an International Journal for Field-Orientated Ichthyology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Length-Weight Relationship and Condition Factor of Six Cichlid (Cichilidae: Perciformis) Species of Anambra River, Nigeria
Journal of Fisheries and Aquaculture ISSN: 0976-9927 & E-ISSN: 0976-9935, Volume 4, Issue 2, 2013, pp.-82-86. Available online at http://www.bioinfopublication.org/jouarchive.php?opt=&jouid=BPJ0000265 LENGTH-WEIGHT RELATIONSHIP AND CONDITION FACTOR OF SIX CICHLID (CICHILIDAE: PERCIFORMIS) SPECIES OF ANAMBRA RIVER, NIGERIA ATAMA C.I.1, OKEKE O.C.1, EKEH F.N.1, EZENWAJI N.E.1, ONAH I.E.1, IVOKE N.1, ONOJA U.S.2 AND EYO J.E.1* 1Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Enugu State, Nigeria. 2Department of Home Science, Nutrition and Dietetics, Faculty of Agriculture, University of Nigeria, Nsukka, Enugu State, Nigeria. *Corresponding Author: Email- [email protected] Received: June 10, 2013; Accepted: July 11, 2013 Abstract- The length-weight relationship and condition factor of six cichlid fish species inhabiting Anambra River were studied. The cichlid fish species were Chromidotilapia guntheri Sauvage 1882, Hemichromis bimaculatus Gill 1862, Tilapia zillii Gervais 1848, Hemichromis fasciatus Peters 1857, Tilapia mariae Boulenger 1899 and Oreochromis niloticus (Linnaeus 1758), obtained from fish landing sites of the river at Oguru- gu, Nsugbe and Otuocha. C. guntheri, H. bimaculatus and T. zillii exhibited positive allometric growth with b = 3.452, 3.828 and 3.210, respec- tively, while H. fasciatus, T. mariae and O. niloticus exhibited negative allometric growth with b = 2.667, 2.272 and 2.792, respectively. There was difference in the condition factors for the combined fish species and the monthly factor for each fish species studied: C. guntheri was 3.44 ± 0.39, H. -

DNA Barcoding Discriminates Freshwater Fishes from Southeastern Nigeria and Provides River System-Level Phylogeographic Resoluti
Mitochondrial DNA, 2011; Early Online: 1–9 DNA barcoding discriminates freshwater fishes from southeastern Nigeria and provides river system-level phylogeographic resolution within some species CHRISTOPHER D. NWANIa, SVEN BECKERb, HEATHER E. BRAIDb, EMMANUEL F. UDEc, OKECHUKWU I. OKOGWUa, & ROBERT HANNERb aDepartment of Applied Biology, Ebonyi State University, Abakaliki, Nigeria, bDepartment of Integrative Biology, Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario, Canada, and cFisheries and Aquaculture, Ebonyi State University, Abakaliki, Nigeria (Received 17 August 2010; revised 28 October 2010; accepted 28 October 2010) Abstract Background and aims: Fishes are the main animal protein source for human beings and play a vital role in aquatic ecosystems and food webs. Fish identification can be challenging, especially in the tropics (due to high diversity), and this is particularly true for larval forms or fragmentary remains. DNA barcoding, which uses the 50 region of the mitochondrial cytochrome c oxidase subunit I (cox1) as a target gene, is an efficient method for standardized species-level identification for biodiversity assessment and conservation, pending the establishment of reference sequence libraries. Materials and methods: In this study, fishes were collected from three rivers in southeastern Nigeria, identified morphologically, and imaged digitally. DNA was extracted, PCR-amplified, and the standard barcode region was bidirectionally sequenced for 363 individuals belonging to 70 species in 38 genera. All specimen provenance data and associated sequence information were For personal use only. recorded in the barcode of life data systems (BOLD; www.barcodinglife.org). Analytical tools on BOLD were used to assess the performance of barcoding to identify species. Results: Using neighbor-joining distance comparison, the average genetic distance was 60-fold higher between species than within species, as pairwise genetic distance estimates averaged 10.29% among congeners and only 0.17% among conspecifics. -

Fish Diversity and Assemblages According to Distance from Source Along a Coastal River Gradient (Ehania River; South- East of Ivory Coast)
Iranian Journal of Fisheries Sciences 14(1)112-129 2015 Fish diversity and assemblages according to distance from source along a coastal river gradient (Ehania River; south- east of Ivory Coast) Konan K.F.1,2,3; Edia O.E.1; Bony K.Y.1,2; Kouamé K.M.1,3; Gourène G.1 Received: May 2013 Accepted: December 2014 Abstract Fish assemblage was investigated during the study of longitudinal profile of the Ehania River Basin in south-eastern Côte d’Ivoire. This area is subjected to intense human activities with many plantations (palm tree, banana, pineapple, coffee, rubber and cocoa). Samples were collected, with gillnets of different mesh sizes, through 6 sampling surveys during dry and rainy seasons from February 2010 to December 2010 at 6 sampling sites. A total of 70 fish species belonging to 48 genera, 28 families and 10 orders were recorded. The temporal variation of diversity index is less marked than spatial variation. The upstream, with 35 species, was less rich in species than the medium area and downstream areas (respectively 46 and 68). The upstream and downstream areas gathered 35 species. Thirty three species were common to the upper and middle areas and 46 species appeared both in the lower courses and the middle area. The 21 species restricted to the lower part of the river are mainly estuarine/marine origin. The beta diversity value revealed low similarity between the lower and upper course of Ehania River. The lowest Downloaded from jifro.ir at 13:43 +0330 on Tuesday October 5th 2021 values of Shannon’s diversity index and equitability index were observed in the middle part of the River which characterized by high population density and intense agricultural activity with many plantations. -

View/Download
CICHLIFORMES: Cichlidae (part 2) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 4.0 - 30 April 2021 Order CICHLIFORMES (part 2 of 8) Family CICHLIDAE Cichlids (part 2 of 7) Subfamily Pseudocrenilabrinae African Cichlids (Abactochromis through Greenwoodochromis) Abactochromis Oliver & Arnegard 2010 abactus, driven away, banished or expelled, referring to both the solitary, wandering and apparently non-territorial habits of living individuals, and to the authors’ removal of its one species from Melanochromis, the genus in which it was originally described, where it mistakenly remained for 75 years; chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), often used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Abactochromis labrosus (Trewavas 1935) thick-lipped, referring to lips produced into pointed lobes Allochromis Greenwood 1980 allos, different or strange, referring to unusual tooth shape and dental pattern, and to its lepidophagous habits; chromis, a name dating to Aristotle, possibly derived from chroemo (to neigh), referring to a drum (Sciaenidae) and its ability to make noise, later expanded to embrace cichlids, damselfishes, dottybacks and wrasses (all perch-like fishes once thought to be related), often used in the names of African cichlid genera following Chromis (now Oreochromis) mossambicus Peters 1852 Allochromis welcommei (Greenwood 1966) in honor of Robin Welcomme, fisheries biologist, East African Freshwater Fisheries Research Organization (Jinja, Uganda), who collected type and supplied ecological and other data Alticorpus Stauffer & McKaye 1988 altus, deep; corpus, body, referring to relatively deep body of all species Alticorpus geoffreyi Snoeks & Walapa 2004 in honor of British carcinologist, ecologist and ichthyologist Geoffrey Fryer (b. -

The Reproductive Biology of the Fishes of Owa Stream, South - West Nigeria
Research Journal of Fisheries and Hydrobiology, 5(2): 81-84, 2010 © 2010, INSInet Publication The Reproductive Biology of the Fishes of Owa Stream, South - West Nigeria. K.B. Olurin and O.I. Odeyemi Department of Plant Science & Applied Zoology, Olabisi Onabanjo University, P.M.B. 2002, Ago-Iwoye, Nigeria. Abstract: Sex ratio, size at maturity, maturity stages, egg diameter and fecundity of some of the fishes of Owa stream, south – west Nigeria were investigated. Only Hemichromis fasciatus differed significantly from the 1:1 sex ratio. Size at maturity for females was 9.05cm for Chromidotilapia guntheri, 10.45cm for Tilapia mariae, and 8.3cm for Hemichromis fasciatus.Mature males were observed for specimens of C.guntheri, while the others had no mature males. The gonado-somatic indices increased with gonadal stage of development, and the values for males were lower than those of the females of corresponding stage of development. Egg diameter was highest in Xenomystus nigri, and least in Ctenoporna kingsleyae. There was a low correlation between fecundity and body weight in C. guntheri. Keywords: Owa stream, gonado – somatic index, size at maturity, sex ratio INTRODUCTION The specimens were sorted out in the laboratory and identified to species level using information Literature is replete with information on the provided by[8]. reproductive biology of the fishes of temperate streams The fishes were mopped on filter paper and their [e.g.,19,1,9,12], with a dearth of information on their total lengths and standard lengths were measured using tropical counterparts. a measuring board to the tenth of a centimeter. -
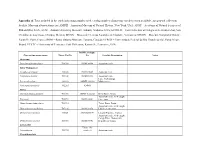
Appendix A. Taxa Included in the Study Indicating Samples Used, Catalog Number of Museum Vouchers When Available, and General Collection Locality
Appendix A. Taxa included in the study indicating samples used, catalog number of museum vouchers when available, and general collection locality. Museum abbreviations are: AMNH – American Museum of Natural History, New York, USA; ANSP – Academy of Natural Sciences of Philadelphia, USA; AUM – Auburn University Museum, Auburn, Alabama, USA; ECOSUR – Fish Collection at Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, Mexico; MCNG – Museo de Ciencias Naturales de Guanare, Venezuela; MNHN – Muséum National d’Histoire Naturelle, Paris, France; ROM – Royal Ontario Museum, Toronto, Canada; UFRGS – Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; UTFTC – University of Tennessee Fish Collection, Knoxville, Tennessee, USA. ROM Catalogue Current taxonomy name Tissue Cat No No Locality Description Notes Outgroup Pseudetroplus maculatus T14743 ROM 98998 Aquarium trade India-Madagascar Etroplus suratensis T13505 ROM 93809 Aquarium trade Paratilapia polleni T13100 ROM 88333 Aquarium trade Lake Andrapongy, Paretroplus damii 201936 AMNH 201936 Madagascar Paretroplus polyactis T12265 AMNH Africa Chromidotilapia guntheri T11700 AMNH I-226361 Beffa River, Benin Aquarium trade, wild caught, Etia nguti T10792 ROM 88042 Cameroon Hemichromis bimaculatus T11719 Tchan Duga, Benin Aquarium trade, wild caught, Heterochromis multidens T07136 ROM 88350 Lobeke, Cameroon Oreochromis niloticus 9092S AMNH254194 Littoral Province, Guinea Aquarium trade, wild caught, Congo River, Democratic Orthochromis stormsi T10766 ROM 88041 Republic of -

Relict Tropical Fish Fauna in Central Sahara
39 Iclitliyol. Explor. Freshwaters, Vol. 1, No. 1, pp. 39-48,2 figs., 1 tab., January 1990 O 1990 by Verlag Dr. Friedrich Pfeil, München, FRG - ISSN 0936-9902 Relict tropical fish fauna in Central Sahara Christian Lévêque * Following a taxonomic revision of freshwater fishes recorded from the Sahara, an updated species list is given. It appears that most species collected in small water bodies are relict populations of widespread species occuring in surrounding river basins, except for Barbus apleurogramina which might be a relict of a more ancient fish fauna. Distribution patterns are discussed in view of the supposed extent of river catchments during the Holocene. Une révision taxinomique des espèces de poissons récoltées dans le Sahara a permis de dresser une liste actualisée prenant en compte les connaissances récentes. I1 apparaît que la plupart des espèces collectées dans les petites collections d'eau sont des populations reliques d'espèces largement répandues dans les bassins hydrographiques voisins, àl'exception de Barbus npleurogranana qui pourrait être un vestige d'une faune plus ancienne. La distribution actuelle est discutée, à la lumière des données sur l'extension supposée des bassins hydrographiques durant l'Holocène. Introduction taxonomic revisions, it turned out that confusion existed in the identificationof some species, often Since the begining of this century, numerous based on small specimens, sometimes poorly Saharan expeditions were able to collect several preserved. The aim of this paper is to give an fish species in the widely scattered and isolated updated taxonomic review of the relict fish small patches of water in this area (Daget, 1959a, populations in the Sahara, and to discuss their 1968; Dumont, 1979, 1987; Estève. -

Diversity, Origin and Intra- Specific Variability
Contributions to Zoology, 87 (2) 105-132 (2018) Monogenean parasites of sardines in Lake Tanganyika: diversity, origin and intra- specific variability Nikol Kmentová1, 15, Maarten Van Steenberge2,3,4,5, Joost A.M. Raeymaekers5,6,7, Stephan Koblmüller4, Pascal I. Hablützel5,8, Fidel Muterezi Bukinga9, Théophile Mulimbwa N’sibula9, Pascal Masilya Mulungula9, Benoît Nzigidahera†10, Gaspard Ntakimazi11, Milan Gelnar1, Maarten P.M. Vanhove1,5,12,13,14 1 Department of Botany and Zoology, Faculty of Science, Masaryk University, Kotlářská 2, 611 37 Brno, Czech Republic 2 Biology Department, Royal Museum for Central Africa, Leuvensesteenweg 13, 3080, Tervuren, Belgium 3 Operational Directorate Taxonomy and Phylogeny, Royal Belgian Institute of Natural Sciences, Vautierstraat 29, B-1000 Brussels, Belgium 4 Institute of Biology, University of Graz, Universitätsplatz 2, A-8010 Graz, Austria 5 Laboratory of Biodiversity and Evolutionary Genomics, Department of Biology, University of Leuven, Ch. Deberiotstraat 32, B-3000 Leuven, Belgium 6 Centre for Biodiversity Dynamics, Department of Biology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway 7 Faculty of Biosciences and Aquaculture, Nord University, N-8049 Bodø, Norway 8 Flanders Marine Institute, Wandelaarkaai 7, 8400 Oostende, Belgium 9 Centre de Recherche en Hydrobiologie, Département de Biologie, B.P. 73 Uvira, Democratic Republic of Congo 10 Office Burundais pour la Protection de l‘Environnement, Centre de Recherche en Biodiversité, Avenue de l‘Imprimerie Jabe 12, B.P. -

Molecular Evolution of Dim-Light Visual Pigments in Neotropical Geophagine Cichlids by Shannon Refvik a Thesis Submitted in Conf
Molecular Evolution of Dim-light Visual Pigments in Neotropical Geophagine Cichlids by Shannon Refvik A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Ecology and Evolutionary Biology University of Toronto c Copyright 2012 by Shannon Refvik Abstract Molecular Evolution of Dim-light Visual Pigments in Neotropical Geophagine Cichlids Shannon Refvik Master of Science Graduate Department of Ecology and Evolutionary Biology University of Toronto 2012 Neotropical cichlid fishes are highly diverse and occupy a wide range of environments. Evo- lution of visual pigments has been important in the diversification of the African rift lake cichlids, but relatively little is known of Neotropical cichlid visual systems. This thesis ad- dresses the molecular evolution of the dim-light visual pigment rhodopsin in the Geophagini tribe of Neotropical cichlids. We use various likelihood-based codon models of molecular evo- lution and newly isolated sequences for Neotropical cichlid rhodopsin to compare patterns of selective constraint among Neotropical, African rift lake, and African riverine cichlid rhodopsin; and provide evidence for differences in selective constraint among clades with positive selection occurring in both the Neotropical and African rift lake clades. We further investigate and find evidence for variation in selective constraint within the geophagine ci- chlids. Comparing the results obtained from different methods suggests that Clade model C is more appropriate than branch-site models for investigating variation in selective con- straint among clades. Neotropical cichlids, alone and in comparison with African cichlids, are emerging as an excellent system for investigating molecular evolution in visual pigments. ii Acknowledgements First and foremost, I would like to thank Hern´anL´opez-Fern´andezand Belinda Chang, my co-supervisors, for their support and advice throughout my degree. -

Diversity and Conservation Status of Fish Fauna from Cavally River in Its
Journal of Entomology and Zoology Studies 2019; 7(3): 1070-1076 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Diversity and conservation status of fish fauna JEZS 2019; 7(3): 1070-1076 © 2019 JEZS from Cavally river in its catchment area under Received: 27-03-2019 Accepted: 29-04-2019 the influence of Gold mining activities (Côte Richard Jean Olive Doffou d’Ivoire) Department of Environment, University Jean Lorougnon Guédé, POB 150 Daloa, Ivory Coast Richard Jean Olive Doffou, Charles Koffi Boussou, Félix Koffi Konan, Gustave N’guessan Aliko and Germain Gourene Charles Koffi Boussou Department of Environment, University Jean Lorougnon Abstract Guédé, POB 150 Daloa, Ivory The present study was conducted from august 2014 to July 2016 in order to assess fish diversity and its Coast conservation status in Cavally river in an area of intensive mining activities. A total of 76 species were recorded including 8 orders, 20 families and 37 genera. Twenty (20) species were registered for the first Félix Koffi Konan time in this river. Characiformes appeared to be the most prolific order in fish population. Among the Department of Environment, species sampled, 3 species (Micralestes eburneensis, Chromidotilapia cavalliensis and Coptodon walteri) University Jean Lorougnon were endemic to the Cavally River. Fish diversity was higher in upstream area of the zone of intensive Guédé, POB 150 Daloa, Ivory mining activities. According to fish conservation status, 52 species were Least Concern (LC), 7 species Coast were Near Threaten (NT), 12 species were Not Evaluated (NE), 4 are Vulnerable and only one species was Data Deficient. Gustave N’guessan Aliko Department of Environment, University Jean Lorougnon Keywords: Cavally, ichthyofauna, diversity, West Africa Guédé, POB 150 Daloa, Ivory Coast Introduction Rivers play a vital role in conservation of biodiversity, the functioning of organisms and the Germain Gourene cycles of organic matter. -

Length-Weight and Length-Length Relationships and Condition Factors of 30 Actinopterygian Fish from the Mono Basin (Benin and Togo, West Africa)
Published in : Cybium (2016), vol. 40, n°4, pp. 267-274 DOI:- Status : Postprint (Author’s version) Length-weight and length-length relationships and condition factors of 30 actinopterygian fish from the Mono basin (Benin and Togo, West Africa) Djiman LEDEROUN1, 2, 3, Philippe LALÈYÈ2, Emmanuel VREVEN3, 4, Pierre VANDEWALLE1 1Laboratory of Functional and Evolutionary Morphology, University of Liège, Chemistry Institute B6, Sart Tilman, B-4000 Liege, Belgium. 2Laboratory of Hydrobiology and Aquaculture, Faculty of Agricultural Sciences, University of Abomey- Calavi, 01 BP: 526 Cotonou, Benin 3Royal Museum for Central Africa, Vertebrate Section, Ichthyology, Leuvensesteenweg 13, 3080 Tervuren, Belgium 4KU Leuven, Laboratory of Biodiversity and Evolutionary Genomics, Charles Deberiotstraat 32, 3000 Leuven, Belgium KEYWORDS: Actinopterygii; West Africa; Mono basin; Length-weight relationship; Length-length relationship; Condition factor ABSTRACT Based on their catches, this study describes the length-weight relationships (LWRs), length- length relationships (LLRs), and condition factors (K) of the 30 most common actinopterygian fish found in the Mono basin. A total of 6,591 specimens were caught, using multiple gears and fishing methods, between February 2011 and May 2014. The allometric b coefficient of the LWRs (BW = a x TLb) ranged between 2.650 for Polypterus senegalus and 3.468 for Awaous lateristriga with an average of 2.993 ± 0.177. Thirteen species had an isometric growth, seven species a negative allometric growth, and for the remaining ten species, growth was positive allometric. Regarding the LLRs, the coefficient of determination r2 was significant and ranged from 0.905 in Petrocephalus bovei to 0.999 in Parachanna obscura with an average of 0.973 ± 0.027. -

Some Aspects of the Reproductive Biology of Tilapia Mariae (Boulenger 1901) in a Small Lake in South- Eastern Nigeria
African Journal of Biotechnology Vol. 7 (14), pp. 2478-2482, 18 July, 2008 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2008 Academic Journals Full Length Research Paper Some aspects of the reproductive biology of Tilapia mariae (Boulenger 1901) in a small lake in south- eastern Nigeria Afamdi Anene1* and Peter U. Okorie2 1Faculty of Agriculture and Vet. Med., Abia State University, Umuahia Campus, P. M. B. 7010, Umuahia, Abia State, Nigeria. 2Department of Animal and Environmental Biology, Imo State University, P.M.B. 2000, Owerri, Imo State, Nigeria. Accepted 14 May, 2008 Some aspects of the reproductive biology of Tilapia mariae of Umuoseriche man-made lake such as fecundity of female fishes was determined gravimetrically after the sex had been established. The ratio of male to female T. mariae of Umuoseriche Lake was 1:1.56. The numbers of female fishes were greater in 110 – 145 mm and 185 – 195 mm size groups. Males dominated the 135 – 175 mm size group. Gonadosomatic index ranged between 1.4 and 3.4 with a mean of 2.25 ± 0.7. Mean ovarian weight was 1.7 ± 0.3 g and a mean fecundity of 1705 ± 629 eggs/ clutch. Key words: Tilapia mariae, gonadosomatic index, fecundity. INTRODUCTION Cichlid fishes have a worldwide distribution but are Omotosho, 1987). There is a dearth of information on the known to have originated from Africa and Madagascar reproductive ability of cichlid fishes in southeast Nigeria. where they are important to the economy and ecology of The only exception is a report on the reproductive biology the ecosystems, which they inhabit.