Quantum Mechanics and Biology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -

Quantum Biology: an Update and Perspective
quantum reports Review Quantum Biology: An Update and Perspective Youngchan Kim 1,2,3 , Federico Bertagna 1,4, Edeline M. D’Souza 1,2, Derren J. Heyes 5 , Linus O. Johannissen 5 , Eveliny T. Nery 1,2 , Antonio Pantelias 1,2 , Alejandro Sanchez-Pedreño Jimenez 1,2 , Louie Slocombe 1,6 , Michael G. Spencer 1,3 , Jim Al-Khalili 1,6 , Gregory S. Engel 7 , Sam Hay 5 , Suzanne M. Hingley-Wilson 2, Kamalan Jeevaratnam 4, Alex R. Jones 8 , Daniel R. Kattnig 9 , Rebecca Lewis 4 , Marco Sacchi 10 , Nigel S. Scrutton 5 , S. Ravi P. Silva 3 and Johnjoe McFadden 1,2,* 1 Leverhulme Quantum Biology Doctoral Training Centre, University of Surrey, Guildford GU2 7XH, UK; [email protected] (Y.K.); [email protected] (F.B.); e.d’[email protected] (E.M.D.); [email protected] (E.T.N.); [email protected] (A.P.); [email protected] (A.S.-P.J.); [email protected] (L.S.); [email protected] (M.G.S.); [email protected] (J.A.-K.) 2 Department of Microbial and Cellular Sciences, School of Bioscience and Medicine, Faculty of Health and Medical Sciences, University of Surrey, Guildford GU2 7XH, UK; [email protected] 3 Advanced Technology Institute, University of Surrey, Guildford GU2 7XH, UK; [email protected] 4 School of Veterinary Medicine, Faculty of Health and Medical Sciences, University of Surrey, Guildford GU2 7XH, UK; [email protected] (K.J.); [email protected] (R.L.) 5 Manchester Institute of Biotechnology, Department of Chemistry, The University of Manchester, -

Biophysics 1
Biophysics 1 on interactions between proteins involved in Notch signaling using BIOPHYSICS modern biophysical methods. Thermodynamics of association and allosteric effects are determined by spectroscopic, ultracentrifugation, http://biophysics.jhu.edu/ and calorimetric methods. Atomic structure information is being obtained by NMR spectroscopy. The ultimate goal is to determine the The Department of Biophysics offers programs leading to the B.A., M.A., thermodynamic partition function for a signal transduction system and and Ph.D. degrees. Biophysics is appropriate for students who wish interpret it in terms of atomic structure. to develop and integrate their interests in the physical and biological sciences. NMR Spectroscopy (Dr. Lecomte) Research interests in the Department cover experimental and Many proteins require stable association with an organic compound computational, molecular and cellular structure, function, and biology, for proper functioning. One example of such “cofactor” is the heme membrane biology, and biomolecular energetics. The teaching and group, a versatile iron-containing molecule capable of catalyzing a research activities of the faculty bring its students in contact with broad range of chemical reactions. The reactivity of the heme group biophysical scientists throughout the university. Regardless of their choice is precisely controlled by interactions with contacting amino acids. of research area, students are exposed to a wide range of problems of Structural fluctuations within the protein are also essential to the fine- biological interest. For more information, and for the most up-to-date list tuning of the chemistry. We are studying how the primary structure of of course offerings and requirements, consult the department web page at cytochromes and hemoglobins codes for heme binding and the motions biophysics.jhu.edu (http://biophysics.jhu.edu/). -

Graduate Group in Biochemistry and Molecular Biophysics General Information for Incoming Students
Graduate Group in Biochemistry and Molecular Biophysics General Information for Incoming Students Student mailboxes are located on the left as you enter the Mail Room (257 Anatomy-Chemistry Building) of the Dept. of Biochemistry and Biophysics. Use the following address to have mail sent to you at this location: Department of Biochemistry and Biophysics, 257 Anatomy-Chemistry Bldg., Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104- 6059. The Student Room is located in 250 Anatomy-Chemistry Building. Keys to this room have been placed in your mailbox. The room has two computers (1 MAC, 1 PC) and a printer. Please make sure the door is locked and securely closed when you leave the room. Lockers: Please contact Kelli McKenna if you would like a locker. They are located in the hallway outside of Rm 234 Anatomy- Chemistry. Advising: You will be meeting individually with the Advising Committee to select your fall courses and for guidance on your rotations. Course registration forms should be returned to Kelli McKenna as soon as possible after your meeting. Any changes in your fall schedule should be made within two weeks of the start of the semester and need to be approved by one of the members of the Advising Committee. Lab Rotation Approval forms are to be completed by Friday, September 13, 2021. You can find the forms for course registration and lab rotation on the BMB webpage: www.med.upenn.edu/bmbgrad under the resources page. Seminars which you are expected to attend: · Raiziss Rounds: Thursdays at noon (Austrian Auditorium, Clinical Research Building). -

BS in Biophysics (285720) MAP Sheet Life Sciences, Cell Biology and Physiology for Students Entering the Degree Program During the 2021-2022 Curricular Year
BS in Biophysics (285720) MAP Sheet Life Sciences, Cell Biology and Physiology For students entering the degree program during the 2021-2022 curricular year. University Core and Graduation Requirements Suggested Sequence of Courses University Core Requirements: FRESHMAN YEAR JUNIOR YEAR Requirements #Classes Hours Classes 1st Semester 5th Semester First-Year Writing or American Heritage 3.0 CELL 360 3.0 Religion Cornerstones CELL 120 (Biological Science) 3.0 CHEM 481 3.0 Teachings and Doctrine of The Book of 1 2.0 REL A 275 CHEM 105 4.0 PHSCS 220 3.0 Mormon MATH 112 (Languages of Learning & Quantitative Reasoning) 4.0 PHSCS 225 2.0 Religion Cornerstone Course 2.0 Religion Elective 2.0 Jesus Christ and the Everlasting Gospel 1 2.0 REL A 250 Total Hours 16.0 Mentored Lab Experience (CELL 495R) 1-2.0 Foundations of the Restoration 1 2.0 REL C 225 2nd Semester Total Hours 14-15.0 The Eternal Family 1 2.0 REL C 200 First-Year Writing or American Heritage 3.0 6th Semester The Individual and Society BIO 250 2.0 CELL 362 3.0 American Heritage 1-2 3-6.0 from approved list CHEM 106 3.0 CELL 363 1.0 CHEM 107 1.0 CHEM 468 3.0 Global and Cultural Awareness 1 3.0 from approved list MATH 113 4.0 Advanced Writing (WRTG 316 recommended) 3.0 Skills Religion Cornerstone Course 2.0 Global & Cultural Awareness Elective 3.0 First Year Writing 1 3.0 from approved list Total Hours 15.0 Religion Elective 2.0 Total Hours 15.0 Advanced Written and Oral Communications 1 3.0 WRTG 316 SOPHOMORE YEAR recommended 3rd Semester SENIOR YEAR MMBIO 240 3.0 7th Semester Quantitative -

An Introductory Treatise on Biophysics - M.I.El Gohary
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS – Vol. III - An Introductory Treatise On Biophysics - M.I.El Gohary AN INTRODUCTORY TREATISE ON BIOPHYSICS M.I.El Gohary Faculty of Science, Al Azhar University, Cairo, Egypt Keywords: Biophysics, interdisciplinary, responsiveness, spermatozoon, budding, irritability, adaptation, mitosis, Auxin, regulator, protozoa, macromolecules, monomers, covalent bonds, aggregate, free energy, hydrocarbons, DNA and RNA, unfold, ribonuclease, catalyst, enzyme-substrate, thiamine, ribosomes, solar energy, adenosine triphosphate, transferase, phosphorylation, photosynthesis, chlorophyll, photophase, dark reaction, anaerobic, aerobic, respiratory pathway, diffusion, random motion, frictional force, macromolecules, random walk, average squared displacement, probability, Fick’s diffusion constant, Fick’s law, inhomogeneous equation, osmosis, semipermeable, osmotic pressure, hypertonic, isotonic, turgor pressure, hydrophobic, hydrophilic, lipid, trilaminar, Myelin, phospholipid, Mosaic, transport, simple diffusion, solute, solubility, electric charge, selective permeability, ion channels, electrochemical gradient, compartment, plasma, sodium pump, permeability, field theory, net flux, selectivity, reversal potential, diffraction, crystallography, Bragg equation, markers, structural organization, detector, magnetic moment, resonance, magnetic dipole, gyromagnetic ratio, the chemical shift, resolution, patch-clamp, food chain, producers, consumers, decomposers, heterotrophic, heterotrophs, biosphere, -

Biophysics and Systems Biology of Cellular Responses to Perturbations
Biophysics and systems biology of cellular responses to perturbations. [email protected] Castellani G.C., Remondini D., Giampieri E., de Oliveira L., Sala C., Menichetti G., Virelli A., Zironi I. Dipartimento di Fisica e Astronomia, Università di Bologna Dipartimento di Fisica Università di Bologna,and INFN Galvani Center for Biophysics, Bioinformatics and Biocomplexity via B.Pichat 6/2 ,Bologna, 40127, Italy IBNS & Physics Department Brown University Providence RI USA Trieste 27-09-2013 Centro Interdipartimentale “L. Galvani” (CIG) Università di Bologna Italy Bologna Center for Biocomplexity 2000: L. Galvani Center for integrated studies of Biophysics Bioinformatics and Biocomplexity ( http://www.centrogalvani.unibo.it ) including Systems Biology, Genomics of Longevity and Laser-Plasma Physics. Bio-immuno-pathological group: expertise in cell culture, sorting and omics measurements: is one of the leading laboratory in Europe and in the World for the study of Aging and senescence in humans, including the centenarians; Biophysics group: decennial activity in the field of complex cellular function modeling and advanced data analysis, and recently is developing biophysical measurements as patch clamp and fluorescence microscopy. An intriguing aspect of Radiation effect is the sharing of mechanisms with cellular senescence, organismic aging and LDR-induced carcinogenesis in humans as: e.g. the Free radical theory (FRT). Harman in the 1950s, . 1) the rate of living theory, which holds that lifespan is an inverse function of metabolic -

Open Position in Biophysics/Chemical Biology
Open position in Biophysics/Chemical Biology Our laboratory of Biophysical Chemistry of Macromolecules (LCBM) at the EPFL in Lausanne, Switzerland (www.epfl.ch/labs/lcbm/) offers a PhD position in the fields of biophysics / chemical biology. Our main focus is to understand the structure and function of chro‐ matin and its role in transcription regulation using a combination of chemistry and biophysics. Recently, our laboratory observed how pioneer transcription factors (pTFs) can access closed chromatin (Mivelaz et al., Molecular Cell 2020). Based on this study and the methods developed therein, we are now investigating molecular mechanisms of chromatin remodeling controlled by pTFs and chromatin remodel‐ ers, using genetics, chemical biology, and single‐molecule biophysics. Within this project, we offer PhD positions, as well as postdoc positions. Your profile (for the PhD position): – A master or equivalent degree in biochemistry, bio‐ physics or chemical biology – Experience in working in a biochemistry or biophysics (imaging) laboratory. – Good knowledge in written and oral English. – Highly motivated for discovering new molecular mechanisms in a challenging environment. – Interest for interdisciplinary projects. – A passion for biophysical chemistry and genetic regulation at the highest level. Application/selection procedure: 1. Send a Letter of motivation, a CV, a summary of previously done research and the contact information of 3 referees to [email protected]. Ideally, we receive your ap‐ plication until March 15, 2021. 2. Selected candidates will be invited for a virtual interview, and, in a second round a virtual visit (including a seminar and meetings with PhD students and postdocs). 3. If you need further information, visit https://lcbm.epfl.ch/fierz/, or contact [email protected]. -

Suggested Guidelines for Starting an Undergraduate Biophysics Program
Suggested Guidelines for Starting an Undergraduate Biophysics Program A biophysics major explores the bridge between biology and physics, applying quantitative methods to solve problems in biology, medicine, and related fields. A biophysics program is interdisciplinary, drawing from coursework in physics, biology, chemistry, mathematics, and statistics. It combines a broad science curriculum with physical and mathematical rigor in prepa- ration for diverse careers. The need for STEM education is well documented. Studies including the influential 2007 National Academies report “Rising Above the Gathering Storm: Energizing and Employing America for a Brighter Economic Future” have warned of potential weaknesses existing in the U.S. STEM education system, and how addressing it relates to national prosperity and power [1]. In the succeeding decade, positive efforts have been made over the entire educational spectrum. Still, there may be a need for over one million more college graduates in STEM fields above the current trajectory in the coming decade [2]. Documented trends indicate the need for interdisciplinary STEM options in particular. For example, the U.S. Department of Labor, Bureau of Labor Statistics projects, from 2014-2024, an increase in employment among interdisciplinary fields like bio- medical engineering (23%), environmental science (11%), and biochemistry/biophysics (8%), above the projected increase for fields like microbiology (4%), physics (7%), and chemistry (3%) [3]. As an interdisciplinary STEM option, a biophysics program brings together faculty from across disciplines, encouraging inter- actions, and potentially leading research and funding opportunities. Further, an undergraduate biophysics program may have a relatively easy implementation, as much of the core coursework (physics, chemistry, biology, and mathematics) is already offered at most institutions. -

Physiology and Biophysics 1
Physiology and Biophysics 1 Physiology and Biophysics Michael D. Cahalan, Department Chair Todd C. Holmes, Department Vice Chair Francesco Tombola, Departmental Graduate Advisor Building D, Room D340, Medical Sciences I 949-824-5864 http://www.physiology.uci.edu/ The Department of Physiology and Biophysics offers research opportunities in molecular biophysics of membranes and proteins, ion channels and signal transduction, molecular and cell biology, structural biology, proteomics, physiological genomics, neuroscience, developmental neurobiology, stem cell biology, endocrinology, cardiac and exercise physiology, GI pathophysiology, immunology, cancer biology, and vision science. The Department offers graduate study under the auspices of the School of Medicine and in conjunction with the graduate program in Cellular and Molecular Biosciences (CMB) and the Interdepartmental Neuroscience Program (INP), which are described in the School of Biological Sciences section (http://catalogue.uci.edu/schoolofbiologicalsciences/#graduatetext). Students are eligible to enter the Department program after meeting the specific requirements of the CMB or INP gateway curriculum or by direct application to the Department. The Department program leads to an M.S. or Ph.D. in Biomedical Sciences, awarded after successful completion of all requirements. Students admitted through either gateway program who select a research advisor in the Department begin following the departmental requirements for the Ph.D. at the beginning of their second year. The faculty conducts quarterly reviews of all continuing students to ensure that they maintain satisfactory progress within their particular academic program. Students participate in a literature review course designed to strengthen research techniques and presentation skills, and attend the monthly Department Colloquium. Students advance to candidacy during the third year; each student presents a seminar on their research projects in preparation for their Ph.D. -

CAMPEP Anatomy and Physiology Requirements As Posted in the Appended CAMPEP Credential Requirements As Follows
WESTERN UNIVERSITY SCHULICH SCHOOL OF MEDICINE & DENTISTRY DEPARTMENT OF MEDICAL BIOPHYSICS BIOPHYS 9706L –CAMPEP PHYSIOLOGY & ANATOMY COURSE OUTLINE: MAY 1- AUGUST 31 2019 DESCRIPTION This course provides a University level background and training in the fundamentals of anatomy and physiology and covers all the major body systems relevant to CAMPEP training requirements for graduate students, including image representation, cadaveric representation and body systems integrating both structure and function. This course concentrates on exposing students to real-world understandings of human physiology and anatomy as it applies to typical diagnostic and therapeutic clinical physics applications. This course was designed to be alligned with and cover all the the CAMPEP requirements for training in human physiology and anatomy for the PhD-MClSc program. CONTACT HOURS: 4 meeting sessions/lectures, self-directed course. PREREQUISITES Undergraduate courses in Engineering and Physics consistent with the CAMPEP program entry requirements. Approved enrollment in CAMPEP PhD-MClSc degree in Medical Biophysics at Western University. Approved enrollment in ARC Bootcamp in fall term following successful completion of this course. ANTIREQUESITES Undergraduate courses in Physiology and Anatomy at Western or any accredited University COURSE CONTENT The following topics will be covered in the following order and timeframe. There will be a meeting session at specific periods and after completion of a body system for the instructor and students to sit face to face together to review previous content and undertake an oral presentation and quiz comprised of a problem or case dedicated to the content. Textbooks: GA = Gray’s Anatomy for Students, 3rd edition; Drake. HP = Human Physiology, an Integrated Approach, 6th edition; Silverthorn. -
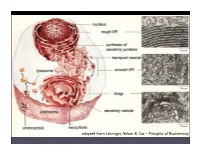
Intro Biophysics
adapted from Lehninger, Nelson & Cox – Principles of Biochemistry Feed: molecules of high energy/low entropy proteins: molecular machines Waste: molecules of low energy/ high entropy assembly of information organised transfer structures adapted from Nelson – Biological Physics order created via energy throughput in a dissipative system: sand ripples created by wind adapted from Nelson – Biological Physics A “simple” cell: E. coli (artist’s rendering after structural/ microscopy data) adapted from Lehninger, Nelson & Cox – Principles of Biochemistry layer 4: layer 3: layer 2: layer 1: cells and organelles supra molecular macromolecules biomolecules complexes nucleotides N~ ---- f o)y! -o-p-o-cko~ The Biological Cell A si;-fil j},. protein amino acids nucleic acids (DNA, etc.) ... only four classes of biomolecules! protein lipid polysaccharide adapted from Phillips et al. – Physical Biology of the Cell Tw o “ polymer languages” are important in biology adapted from Phillips et al. – Physical Biology of the Cell Translation between the “polymer languages”: The Genetic Code adapted from Phillips et al. Physical Biology of the Cell adapted from Phillips / Kondev / Theriot – Physical Biology of the Cell model building in biophysics: biological cartoons represent idealizations of different aspects of relevance in different contexts adapted from Phillips et al. – Physical Biology of the Cell adapted from Phillips et al. – E. coli Physical Biology of the Cell 3 cell volume VE coli 1 μm cell mass m 1 pg E coli biology by numbers: repl cycle time tE coli 3,000 s order-of-magnitude 2 surface area AE coli 6 μm estimates are essential 6 for model building! genome length NE coli 5×10 bp swimming speed vE coli 20 μm/s double-stranded DNA length per bp lbp 0.34 nm 3 volume per bp Vbp 1 nm charge density per unit length λDNA 2 e/0.34 nm persistence length ξDNA 50 nm amino acids and proteins typical diameter dprotein 4–5 nm 3 typical volume Vprotein 25 nm avrg.