FROM MALIR RIVER WITHIN KARACHI Raheela Sharmeen M.Sc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SEF Assisted Schools (SAS)
Sindh Education Foundation, Govt. of Sindh SEF Assisted Schools (SAS) PRIMARY SCHOOLS (659) S. No. School Code Village Union Council Taluka District Operator Contact No. 1 NEWSAS204 Umer Chang 3 Badin Badin SHUMAILA ANJUM MEMON 0333-7349268 2 NEWSAS179 Sharif Abad Thari Matli Badin HAPE DEVELOPMENT & WELFARE ASSOCIATION 0300-2632131 3 NEWSAS178 Yasir Abad Thari Matli Badin HAPE DEVELOPMENT & WELFARE ASSOCIATION 0300-2632131 4 NEWSAS205 Haji Ramzan Khokhar UC-I MATLI Matli Badin ZEESHAN ABBASI 0300-3001894 5 NEWSAS177 Khan Wah Rajo Khanani Talhar Badin HAPE DEVELOPMENT & WELFARE ASSOCIATION 0300-2632131 6 NEWSAS206 Saboo Thebo SAEED PUR Talhar Badin ZEESHAN ABBASI 0300-3001894 7 NEWSAS175 Ahmedani Goth Khalifa Qasim Tando Bago Badin GREEN CRESCENT TRUST (GCT) 0304-2229329 8 NEWSAS176 Shadi Large Khoski Tando Bago Badin GREEN CRESCENT TRUST (GCT) 0304-2229329 9 NEWSAS349 Wapda Colony JOHI Johi Dadu KIFAYAT HUSSAIN JAMALI 0306-8590931 10 NEWSAS350 Mureed Dero Pat Gul Mohammad Johi Dadu Manzoor Ali Laghari 0334-2203478 11 NEWSAS215 Mureed Dero Mastoi Pat Gul Muhammad Johi Dadu TRANSFORMATION AND REFLECTION FOR RURAL DEVELOPMENT (TRD) 0334-0455333 12 NEWSAS212 Nabu Birahmani Pat Gul Muhammad Johi Dadu TRANSFORMATION & REFLECTION FOR RURAL DEVELOPMENT (TRD) 0334-0455333 13 NEWSAS216 Phullu Qambrani Pat Gul Muhammad Johi Dadu TRANSFORMATION AND REFLECTION FOR RURAL DEVELOPMENT (TRD) 0334-0455333 14 NEWSAS214 Shah Dan Pat Gul Muhammad Johi Dadu TRANSFORMATION AND REFLECTION FOR RURAL DEVELOPMENT (TRD) 0334-0455333 15 RBCS002 MOHAMMAD HASSAN RODNANI -

Lakeside Energy (Pr Vate) Limited
BEFORE THE NATIONAL elECTRIC POWER REGULATORY AUTHORITY APPLICATION FOR GENERATION LICENCE IN RESPECT OF 50 MW WIND POWER PROJECT IN JHIMPIR, DISTRICT THATTA, PROVINCE OF SINDH, PAKISTAN Dated: 25.05.2017 Filed for and behalf of: LAKESIDE ENERGY (PR VATE) LIMITED Through: RIAA BARKER GILLETTE 68, NAZIMUDDIN ROAD, F-8/4, ISLAMABAD UAN: (051) 111-LAWYER WEBSITE: www.riaabarkergillette.com 31.05.2017 Registrar National Electric Power Regulatory Authority NEPRA Tower, Ataturk Avenue (East), Sector G-5/1 Islamabad, Pakistan. SUBJECT: APPLICATION FOR GRANT OF GENERATION LICENCE I, Brigadier (Retired) Tariq Izaz, Chief Operating Officer, being the duly authorized representative of Lakeside Energy {Private} Limited by virtue of a board resolution dated 15.01.2017, hereby to the National Electric Power Regulatory Authority {NEPRA} for the grant of a generation licence to pursuant to section 15 of the Regulation of Generation, Transmission and Distribution of Electric Power Act, 1997. I certify that the documents-in-support attached with this application are prepared and submitted in conformity with the provision of National Electric Power Regulatory Authority (NEPRA) Licensing (Application and Modification Procedure) Regulations, 1999, and undertake to abide by the terms and provisions of above-said regulations. I further undertake and confirm that the information provided in the attached documents-in-support is true and corrected to the best of my knowledge and belief. A pay order in the sum of Rupees Three Hundred Thousand Three Thirty Six (PKR 300,336/-), being the non-refundable application processing fee calculated in accordance with Schedule-ll to National Electric Power Regulatory Authority Licensing (Application and Modification Procedure) Regulations, 1999, is also attached herewith. -

Malir-Karachi
Malir-Karachi 475 476 477 478 479 480 Travelling Stationary Inclass Co- Library Allowance (School Sub Total Furniture S.No District Teshil Union Council School ID School Name Level Gender Material and Curricular Sport Total Budget Laboratory (School Specific (80% Other) 20% supplies Activities Specific Budget) 1 Malir Karachi Gadap Town NA 408180381 GBLSS - HUSSAIN BLAOUCH Middle Boys 14,324 2,865 8,594 5,729 2,865 11,459 45,836 11,459 57,295 2 Malir Karachi Gadap Town NA 408180436 GBELS - HAJI IBRAHIM BALOUCH Elementary Mixed 24,559 4,912 19,647 4,912 4,912 19,647 78,588 19,647 98,236 3 Malir Karachi Gadap Town 1-Murad Memon Goth (Malir) 408180426 GBELS - HASHIM KHASKHELI Elementary Boys 42,250 8,450 33,800 8,450 8,450 33,800 135,202 33,800 169,002 4 Malir Karachi Gadap Town 1-Murad Memon Goth (Malir) 408180434 GBELS - MURAD MEMON NO.3 OLD Elementary Mixed 35,865 7,173 28,692 7,173 7,173 28,692 114,769 28,692 143,461 5 Malir Karachi Gadap Town 1-Murad Memon Goth (Malir) 408180435 GBELS - MURAD MEMON NO.3 NEW Elementary Mixed 24,882 4,976 19,906 4,976 4,976 19,906 79,622 19,906 99,528 6 Malir Karachi Gadap Town 2-Darsano Channo 408180073 GBELS - AL-HAJ DUR MUHAMMAD BALOCH Elementary Boys 36,374 7,275 21,824 14,550 7,275 29,099 116,397 29,099 145,496 7 Malir Karachi Gadap Town 2-Darsano Channo 408180428 GBELS - MURAD MEMON NO.1 Elementary Mixed 33,116 6,623 26,493 6,623 6,623 26,493 105,971 26,493 132,464 8 Malir Karachi Gadap Town 3-Gujhro 408180441 GBELS - SIRAHMED VILLAGE Elementary Mixed 38,725 7,745 30,980 7,745 7,745 30,980 123,919 -
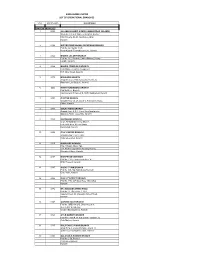
Sindh Bank Limited List of Operational Branches
SINDH BANK LIMITED LIST OF OPERATIONAL BRANCHES S.No. Branch Code Branch Name KARACHI BRANCHES 1 5303 ALLAMA SHABBIR AHMED USMANI ROAD (ISLAMIC) Shop No.2,3, & 4, Shaheen Heights, Block-7, KDA Scheme No.24, Gulshan-e-Iqbal, Karachi 2 0329 BUFFERZONE NAGAN CHOWRANGI BRANCH Plot No. 32, Sector 11-H, North Karachi Township Scheme, Karachi 3 0364 BHAINS COLONY BRANCH Plot No. 217, Block-A, Cattle (Bhains) Colony Landhi, Karachi 4 0366 BAHRIA COMPLEX-II BRANCH Plot # Misc.-2, Bahria Complex-II M.T. Khan Road, Karachi 5 0375 BOHRAPIR BRANCH Shop No.3 & 4, Plot Survey No.88, RC.12 Ranchore Line Quarter, Karachi 6 0355 NORTH NAZIMABAD BRANCH Plot No.B-65, Block-L, Improvement Scheme # 2, North Nazimabad, Karachi 7 0302 CLIFTON BRANCH Ground Floor, St-28, Block-5, Federation House, Clifton, Karachi 8 0303 COURT ROAD BRANCH Ground floor, G-5-A, Court View Apartments, Opposite Sindh Assembly, Karachi 9 0368 KARIMABAD BRANCH C-23, Al-Habib Blessing, Block-4, Federal B Area, Meena Bazar, Karimabad, Karachi 10 0369 CIVIC CENTER BRANCH Ground Floor, Civic Center, Gulshan-e-Iqbal, Karachi 11 0325 DHORAJEE BRANCH Plot # 35/243, Block 7&8, C.P. Berar Cooperative Housing Society, Dhorajee Colony, Karachi 12 0357 DHA PHASE-II BRANCH Plot No. 13-C, Commercial Area “A”, DHA Phase-II, Karachi 13 0387 GADAP TOWN BRANCH Plot No.345, Haji Zakaria Jokhio Goth, Distt. Malir, Karachi 14 0304 DHA 26th STREET BRANCH Plot No.14-E, 26th Street Phase 5 Ext. D.H.A, Karachi 15 0372 DR. ZIAUDDIN AHMED ROAD Plot No.2/1, Sheet No. -

Iffice of the DISTRICT MUNICIPAL CORPORATION, MALIR KARACHI No: DMC (Malir) /Administrator/ �7 /15� Dated / 1 001 — 2015
iFFICE OF THE DISTRICT MUNICIPAL CORPORATION, MALIR KARACHI No: DMC (Malir) /Administrator/ 7 /15 Dated / 1 001 — 2015 The Director (CB), SPPRA Karachi, SUBMISSION OF BID EVALUATION REPORTS OF NIT NUMBER DMC/MALIR/G.ZONE /ADMINISTRATOR/42/2014 DATED 02/02/2015 tSR NO. 22640), Please find enclosed herewith bid evaluation reports for the works/tenders which were invited through the NIT mentioned in the above noted subject for your information and record. c A MIN STRA TOR DISTRICT MUNICIPAL CORPORATION MALIR, KARACHI DISTRICT MUNICIPAL CORPORATION MALIR KARACHI (GADAP ZONE) I. Bid Evaluation Report -05 Works 1 Name of Procuring Agency: District Municipal Corporation Malir Karachi Gadap Zone No. DMC Malir / G.Zone/Administrator/042/2014, 2 Tender Reference No dated 02-02-2015. Tender Description/name of Work / Supply of Man hole covers & Ring Slab at Union Council 3 item Murad Memon Gadap Zone . 4 Method of Procurement Single Stage (one envelop) 5 Tender Published: Through SPPRA website 6 Total Bid documents Sold: 03 Nos 7 Total Bids Received 03 Nos Technical Bid Opening date 8 N/A (if applicable): No. of Bid technically qualified 9 N/A (if applicable) 10 Bid(s) Rejected 01 No. (2% E.M not attached) 11 Financial Bid Opening date 23-02-2015 1. Bid Evaluation Report: Cost Ranking Comparison Reasons for Name of Firm S.No offered by in terms of with Estimated acceptance/ Remarks or Bidder the Bidder cost cost rejection 1 2 3 4 5 6 7 Pay order No. 8136797 M/S KB Substantially 9,89,3941= Im lowest HAW•Move . -

An Assessment of Pollution in Major Creeks Around Port Qasim
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aquatic Commons Pakistan Journal of Marine Sciences, Vol. 18(1&2), 1-9, 2009. AN ASSESSMENT OF POLLUTION IN MAJOR CREEKS AROUND PORT QASIM Muhammad Uzair Khan, Kanwal Nazim, Moinuddin Ahmed, Syed Shahid Shaukat, Qadeer Mohammad Ali, Agha Tahir Hussain Durrani, Wafra Matanat Zaheen Marine Reference Collection and Resource Centre, University of Karachi, Pakistan (MUK, KN, QMA, WMZ); Laboratory of Dendrochronology and Plant Ecology, Department of Botany, Federal Urdu University of Arts, Science & Technology Gulshan-e-Iqbal, Karachi, Pakistan (MA, SSS); Sindh Forest Department, Government of Sindh (ATHD). e-mail: [email protected] ABSTRACT: Discharge of industrial and domestic wastes in sea alter the physical and chemical properties of sea water, which may affect the marine ecosystem. This study was aimed to measure the water quality of eight different creeks around Port Qasim. Samples were collected and analyzed using standard procedures during February and March, 2009. The parameters including pH, Conductivity, Total Dissolved Solids, Salinity, Dissolved Oxygen, Nitrate, Phosphate, Sulphate, Chloride, Calcium, Potassium, Zinc, Iron, Cobalt, Chromium, Manganese and Cadmium were determined. The result shows that Bakran creek and Gharo-Phitti creeks are heavily polluted compared to other creeks. This study recommends that wastes from the industries or sewerage should be treated before being discharge into the creek system. Key words: Creeks, Port Qasim, nutrients, Gharo-Phitti, Bakran INTRODUCTION Pollution of water bodies is a major concern in the developing nations. According to various workers (Fakayode, 2005; Emongor et al., 2005; Furtado et al., 1998; Ugochukwu, 2004; Altman and Parizek, 1995) anthropogenic activities i.e. -

State Bank of Pakistan I.I. Chundrigarh Road Karachi KHI001 United Bank Ltd
State Bank of Pakistan I.I. Chundrigarh Road Karachi KHI001 United Bank Ltd. NEAR BALOCH COLONY BUS STOP Karachi KHI003 United Bank Ltd. RAFIH E AAM SOCIETY Karachi KHI004 BankIslami Ltd. DHA PHASE-2 Karachi KHI005 Allied Bank Ltd. SCHEME-36,GULISTAN-E-JOHAR, UNI ROAD Karachi KHI006 United Bank Ltd. STOCK EXCHANGE BLDG.I.I.CHUNDRIGAR Karachi KHI007 Allied Bank Ltd. SADDAR BAZAR QUARTER, ZAIB UN NISA St Karachi KHI008 Allied Bank Ltd. WEST WHARF DOCKYARD ROAD, Karachi KHI009 Askari Bank Ltd. ASKARI BANK LTD., MALIR CANTT BRANCH Karachi KHI010 Faysal Bank Ltd. Q-14, Sector 33-A, Korangi # 2 (209) Karachi KHI011 HBL LASBELA Karachi KHI012 HBL SHIREEN JINNAH Karachi KHI013 Habib Metropolitan North Western Zone Prt Qasim Karachi KHI014 Bank Ltd. Askari Bank Ltd. Guslah-e-Iqbal Branch Karachi KHI015 Meezan Bank Ltd. Opp Jungle Shah College, Kemari Town Karachi KHI016 Bank Al Habib Ltd. Malir Halt Railway Stn,Shahrah Faisal Karachi KHI017 Bank Al Habib Ltd. Near Siemens Chowrangi, S.I.T.E Karachi KHI018 Silk Bank Ltd. KCHS bahadurabad karachi Karachi KHI019 Bank Al Habib Ltd. Rashid Minhas,Block B Gulshan-e-Jamal Karachi KHI020 Bank Al Habib Ltd. Speedy Towers, Phase-I, DHA Karachi KHI021 Bank Al Habib Ltd. Block 17 KDA36,Shalimar Center,Jauhar Karachi KHI022 BankIslami Ltd. 13-C, GULSHAN E IQBAL UNIVERSIITY ROAD Karachi KHI023 BankIslami Ltd. I I CHUNDRIGAR ROAD, Karachi KHI024 Bank Al Habib Ltd. Block 13A, Uni Road Gulshan Iqbal Karachi KHI025 BankIslami Ltd. BLOCK-15 KDA SCHEME 36,GULISTAN-E-JOHER Karachi KHI026 Faysal Bank Ltd. ST - 02, Main Shahrah-e-Faisal (110) Karachi KHI027 MCB Bank Ltd MCB Shah Faisal Colony Branch No. -

Development of High Speed Rail in Pakistan
TSC-MT 11-014 Development of High Speed Rail in Pakistan Stockholm, June 2011 Master Thesis Abdul Majeed Baloch KTH |Development of High Speed Rail In Pakistan 2 Foreword I would like to express my sincere gratitude to my supervisors, Anders Lindahl, Bo-Lennart Nelldal & Oskar Fröidh for their encouragement, patience, help, support at different stages & excellent guidance with Administration, unique ideas, feedback etc. Above all I would like to thank my beloved parents ’Shazia Hassan & Dr. Ali Hassan’ , my brothers, sisters from soul of my heart, for encouragement & support to me through my stay in Sweden, I wish to say my thanks to all my friends specially ‘ Christina Nilsson’ for her encouragement, and my Landlord ‘Mikeal & Ingmarie’ in Sweden . Finally I would like to say bundle of thanks from core of my Heart to KTH , who has given me a chance for higher education & all people who has been involved directly or in-directly with completion of my thesis work Stockholm, June 2011 Abdul Majeed Baloch [email protected] KTH |Development of High Speed Rail In Pakistan 3 KTH |Development of High Speed Rail In Pakistan 4 Summary Passenger Railway service are one of the key part of the Pakistan Railway system. Pakistan Railway has spent handsome amount of money on the Railway infrastructure, but unfortunately tracks could not be fully utilized. Since last many years due to the fall of the Pakistan railway, road transport has taken an advantage of this & promised to revenge. Finally road transport has increased progressive amount of share in his account. In order to get the share back, in 2006 Pakistan Railway decided to introduce High speed train between Rawalpindi-Lahore 1.According Pakistan Railway year book 2010, feasibility report for the high speed train between Rawalpindi-Lahore has been completed. -

Government of Sindh Road Resources Management (RRM) Froject Project No
FINAL REPORT Mid-Term Evaluation /' " / " kku / Kondioro k I;sDDHH1 (Koo1,, * Nowbshoh On$ Hyderobcd Bulei Pt.ochi 7 godin Government of Sindh Road Resources Management (RRM) Froject Project No. 391-0480 Prepared for the United States Agency for International Development Islamabad, Pakistan IOC PDC-0249-1-00-0019-00 * Delivery Order No. 23 prepared by DE LEUWx CATHER INTERNATIONAL LIMITED May 26, 1993 Table of Contents Section Pafle Title Page i Table of Contents ii List of Tables and Figures iv List of Abbieviations, Acronyms vi Basic Project Identification Data Sheet ix AID Evaluation Summary x Chapter 1 - Introduction 1-1 Chapter 2 - Background 2-1 Chapter 3 - Road Maintenance 3-1 Chapter 4 - Road Rehabilitation 4-1 Chapter 5 - Training Programs 5-1 Chapter 6 - District Revenue Sources 6-1 Appendices: - A. Work Plan for Mid-term Evaluation A-1 - B. Principal Officers Interviewed B-1 - C. Bibliography of Documents C-1 - D. Comparison of Resources and Outputs for Maintenance of District Roads in Sindh D-1 - E. Paved Road System Inventories: 6/89 & 4/93 E-1 - F. Cost Benefit Evaluations - Districts F-1 - ii Appendices (cont'd.): - G. "RRM" Road Rehabilitation Projects in SINDH PROVINCE: F.Y.'s 1989-90; 1991-92; 1992-93 G-1 - H. Proposed Training Schedule for Initial Phase of CCSC Contract (1989 - 1991) H-1 - 1. Maintenance Manual for District Roads in Sindh - (Revised) August 1992 I-1 - J. Model Maintenance Contract for District Roads in Sindh - August 1992 J-1 - K. Sindh Local Government and Rural Development Academy (SLGRDA) - Tandojam K-1 - L. -

Government of Sindh Finance Department
2021-22 Finance Department Government of Sindh 1 SC12102(102) GOVERNOR'S SECRETARIAT/ HOUSE Rs Charged: ______________ Voted: 51,652,000 ______________ Total: 51,652,000 ______________ ____________________________________________________________________________________________ GOVERNOR'S SECRETARIAT ____________________________________________________________________________________________ BUILDINGS ____________________________________________________________________________________________ P./ADP DDO Functional-Cum-Object Classification & Budget NO. NO. Particular Of Scheme Estimates 2021 - 2022 ____________________________________________________________________________________________ Rs 01 GENERAL PUBLIC SERVICE 011 EXECUTIVE & LEGISLATIVE ORGANS, FINANCAL 0111 EXECUTIVE AND LEGISLATIVE ORGANS 011103 PROVINCIAL EXECUTIVE KQ5003 SECRETARY (GOVERNOR'S SECRETARIAT/ HOUSE) ADP No : 0733 KQ21221562 Constt. of Multi-storeyed Flats Phase-II at Sindh Governor's 51,652,000 House, Karachi (48 Nos.) including MT-s A12470 Others 51,652,000 _____________________________________________________________________________ Total Sub Sector BUILDINGS 51,652,000 _____________________________________________________________________________ TOTAL SECTOR GOVERNOR'S SECRETARIAT 51,652,000 _____________________________________________________________________________ 2 SC12104(104) SERVICES GENERAL ADMIN & COORDINATION Rs Charged: ______________ Voted: 1,432,976,000 ______________ Total: 1,432,976,000 ______________ _____________________________________________________________________________ -

Senate Secretariat Orders of the Day
SENATE SECRETARIAT ORDERS OF THE DAY for the meeting of the Senate to be held at 02:00 p.m. on Wednesday, the 23rd November, 2016. 1. Recitation from the Holy Quran. QUESTIONS 2. Questions entered in a separate list to be asked and answers given. CONSIDERATION OF ADMISSIBILITY OF ADJOURNMENT MOTIONS 3. Admissibility of the following Adjournment Motions will be determined:- (i) Adj. Motion No. 1 (6-255/2016-M), notice given of by Senator Col. (R) Syed Tahir Hussain Mashhadi to discuss the unfortunate incident of bomb blast at Civil Hospital, Quetta which resulted into loss of nearly hundred lives and injuries to over one hundred and fifty people. (ii) Adj. Motion No. 2 (12-255/2016-M), notice given of by Senator Dr. Jehanzeb Jamaldini, to discuss the Tobacco Industry’s advertisement, promotion, sponsorship and CSR (Corporate Social Responsibility) i.e. funding in charity to cover up the fact that they sell deadly products. (iii) Adj. Motion No. 3 (16-255/2016-M), notice given of by Senator Sirajul Haq to discuss the report of the Auditor General of Pakistan for the year 2014-15 in which the financial irregularities of 182 billion rupees has been pointed out in Federal Board of Revenue’s Inland Revenue Service. CALLING ATTENTION NOTICE 4. SENATORS SHERRY REHMAN, MUKHTIAR AHMED DHAMRAH @ AAJIZ, KARIM AHMED KHAWAJA, SALEEM MANDVIWALA AND SAEEDUL HASSAN MANDOKHAIL to draw attention of the Minister for Railways, towards the train accident at Landhi Railway Station which resulted into loss of more than twenty precious lives of innocent citizens and injuries to many. -

Active Franchise List ~ NADRA.Xlsx
NADRA e-Sahulat outlets ISLAMABAD 1 Bilal traders shop no 7 ground floor mughal market I‐8/1 2 Islamabad Karachi company G ‐ 9 Markaz NADRA Kisok 3 Subicca Inc. H.No. 112 Street No 70 Opposite Filter Plant G 9/3 Islamabad. 4 Pearl Iceream,Yammy Icecream franchise,Shop No. B‐2,Shaheen Plaza,near Utility Sotre,I‐10 Markaz 5 Al Rehmat enterprises Al rehmat market shops 01‐03 street no 4 koral town Islamabad 6 Office No. 6, First Floor, Malik Plaza, Block 16‐C, Upper Floor NRC F‐8, Islamabad 7 Friends Computers Shop no 9 basement Farhan Plaza G‐11 Markaz Islamabad 8 Arslan Hardware Electric & Paint Store machine stop Darkala road chowk Harno Thanda Pani Islamabad 9 Pakistan Town, Phase‐I near Lohi Bheer 10 Tariq Market F‐10/2. 11 Shop No. 1‐B Plot No. 2/A Pakeza Market I‐8/4 Islamabad 12 Hamza Card & Mobile, Saifullah Plaza, Rawat 13 Basement floor hajveri plaza 22‐b chaina chock blue area F‐6,G‐6 14 Adeel Mobile Shop & Call Customer Services Bharak Kahu 15 Main CDA Headquarters Building Compound 16 Aamir Communication Jabba UC Tarlai 17 Al‐Rehman Stationary Golra Sharif 18 Arsalan Customer Service, PCO, Nei Abadi Korang Sohan Warsi Market Islamabad. 19 Dhanyal Customer Near Police Station Service Simly Dam Road Near Barakahu. 20 Moh Usman Abad Near Masjid Abdullah Bin Zubair Bhara Kaho Islamabad 21 Village Chatta Bakhtawar Islamabad. 22 Shop No.8, Mughal Market, Irfanabad, P.O Tarlai Kalan, Taramri Chowk. 23 Main Bazar Sang Jani, Tehsil & Distt Islamabad 24 Village Farash Near Girls High School Federal Area Islamabad.