Molecular Basis of Dark Adaptation in Rod Photoreceptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
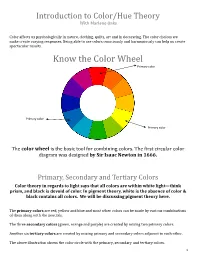
Know the Color Wheel Primary Color
Introduction to Color/Hue Theory With Marlene Oaks Color affects us psychologically in nature, clothing, quilts, art and in decorating. The color choices we make create varying responses. Being able to use colors consciously and harmoniously can help us create spectacular results. Know the Color Wheel Primary color Primary color Primary color The color wheel is the basic tool for combining colors. The first circular color diagram was designed by Sir Isaac Newton in 1666. Primary, Secondary and Tertiary Colors Color theory in regards to light says that all colors are within white light—think prism, and black is devoid of color. In pigment theory, white is the absence of color & black contains all colors. We will be discussing pigment theory here. The primary colors are red, yellow and blue and most other colors can be made by various combinations of them along with the neutrals. The three secondary colors (green, orange and purple) are created by mixing two primary colors. Another six tertiary colors are created by mixing primary and secondary colors adjacent to each other. The above illustration shows the color circle with the primary, secondary and tertiary colors. 1 Warm and cool colors The color circle can be divided into warm and cool colors. Warm colors are energizing and appear to come forward. Cool colors give an impression of calm, and appear to recede. White, black and gray are considered to be neutral. Tints - adding white to a pure hue: Terms about Shades - adding black to a pure hue: hue also known as color Tones - adding gray to a pure hue: Test for color blindness NOTE: Color theory is vast. -

Shedding New Light on the Generation of the Visual Chromophore PERSPECTIVE Krzysztof Palczewskia,B,C,1 and Philip D
PERSPECTIVE Shedding new light on the generation of the visual chromophore PERSPECTIVE Krzysztof Palczewskia,b,c,1 and Philip D. Kiserb,d Edited by Jeremy Nathans, Johns Hopkins University School of Medicine, Baltimore, MD, and approved July 9, 2020 (received for review May 16, 2020) The visual phototransduction cascade begins with a cis–trans photoisomerization of a retinylidene chro- mophore associated with the visual pigments of rod and cone photoreceptors. Visual opsins release their all-trans-retinal chromophore following photoactivation, which necessitates the existence of pathways that produce 11-cis-retinal for continued formation of visual pigments and sustained vision. Proteins in the retinal pigment epithelium (RPE), a cell layer adjacent to the photoreceptor outer segments, form the well- established “dark” regeneration pathway known as the classical visual cycle. This pathway is sufficient to maintain continuous rod function and support cone photoreceptors as well although its throughput has to be augmented by additional mechanism(s) to maintain pigment levels in the face of high rates of photon capture. Recent studies indicate that the classical visual cycle works together with light-dependent pro- cesses in both the RPE and neural retina to ensure adequate 11-cis-retinal production under natural illu- minances that can span ten orders of magnitude. Further elucidation of the interplay between these complementary systems is fundamental to understanding how cone-mediated vision is sustained in vivo. Here, we describe recent -

Original Or Non-Original Expressive Art Guide
Expressive Art Oregon 4-H Tip Sheet OriginalArts or Non-original Art Guide Does the work use design components created by someone else? (patterns, drawings, recognizable pictures or photos, stencils, stamps, pre-shaped forms) NO YES Original Art Division Non-original Art Division Member applies the elements and principles of design Member applies the elements and principles of to create work that is entirely their own design to create work that may incorporate pieces designed or created by others. Pre-designed component must not be the total design Drawing & Sketching (by technique) Drawing/Shading Techniques • Line Drawing Uses drawing, shading, texturing and/or three Uses any drawing medium that can make a distinct line dimensional shaping techniques with the aid of partial Examples include pencil, colored pencil, scratch art, pen and photographs, line drawings, tracing or stenciling that ink, felt tip markers the member did not create themselves Differences in lightness/darkness can be achieved by spacing Includes soft metal embossing, woodburning, scratch or thickness of distinct lines art, or drawing to complete or enhance a partial photo • Shaded Drawing of a subject Uses any drawing medium that can make varied differences in lightness and darkness without showing distinct lines Shading is not simply adding color, it is a technique that adds dimension or volume to the piece. It introduces degrees of darkness to render light and shadow. Examples include chalk, charcoal, pastels, pencil, and colored pencil • Line & Shaded Combination -

Darkness and Light: the Day of the Lordi
BIBLICAL PROPHECY—THINGS TO COME Darkness and light: The Day of the Lordi Now, brothers, about times and dates we do not need to write to you, for you know very well that the day of the Lord will come like a thief in the night. While people are saying, "Peace and safety," destruction will come on them suddenly, as labor pains on a pregnant woman, and they will not escape. But you, brothers, are not in darkness so that this day should surprise you like a thief. You are all sons of the light and sons of the day. We do not belong to the night or to the darkness. (1 Thessalonians 5:1-5) In these verses believers are called ―brothers.‖ Those who are saying, ―Peace and safety,‖ are unbelievers. God is reminding the Thessalonians that unbelievers will not escape judgment in the ―Day of the Lord.‖ However, believers are not in darkness, they are ―sons of the light,‖ sons of faith in Christ, and can look back on the accomplished salvation of Christ, which fulfilled Old Testament promises. They can look forward to the second coming of Christ, in the Day of the Lord, which consummates all of God’s prophecy/promises. The Day of the Lord was the high hope and the far-off goal of the Old Testament. It was, that toward which, the entire Old Testament program of God was moving. Everything in time and creation looked forward to and moved toward that day. The Old Testament era closed without it being realized, and up to today the Day of the Lord has not yet come. -

Color Wheel Value Scale
Name Date Period ART STUDENT LEARNING GUIDE: Color Wheel Value scale ESSENTIAL QUESTIONS . I know and understand the answers to the following questions: Pre-Project Post-Project Yes / N0 Yes / No What are the properties of the primary colors? How do you create secondary & tertiary/intermediary colors? How do you create tints & shades ? How do you locate the complementary colors? What are their properties? What is the difference between warm & cool colors? LEARNING TARGETS . 1. I can create a Color Wheel by mixing primary paint colors & adding black & white to create different Values (tints & shades). 2. I can construct a Color Wheel by using the following Element of Art: Color 3. I can construct a Color Wheel by using the following art techniques: Value (tints & shades) 4. I can analyze and evaluate the merit of my completed artwork by completing a student self-assessment. 5. I can thoughtfully plan and successfully create an artwork by staying engaged in class and being on-task at all times. KEY VOCABULARY . Color wheel – a tool used by artists to see how colors work together Primary colors – Red, Yellow, Blue (cannot be created by any other colors) Secondary colors – Orange, Violet/Purple, Green (created by combing two primary colors) Tertiary/Intermediary colors – created by combing a secondary color and a primary color (ex. Yellow-green) Warm colors – red, orange, yellow Cool colors – blue, green, purple Complementary colors – colors opposite of each other on the color wheel ( next to each other they appear brighter, mixed together they create brown) Tints – adding white to a color Shades- adding black to a color Value – the lightness or darkness of a color (tints and shades) Hue – pure color (no white or black added) Created by A. -

ATP Consumption by Mammalian Rod Photoreceptors in Darkness and in Light
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 18, 1917–1921, December 23, 2008 ª2008 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2008.10.029 Report ATP Consumption by Mammalian Rod Photoreceptors in Darkness and in Light Haruhisa Okawa,1 Alapakkam P. Sampath,2 transduction. To calculate the energy required to pump out Simon B. Laughlin,3 and Gordon L. Fain4,* Na+ entering through the channels, which must be removed 1Neuroscience Graduate Program to keep the cell at steady state, we assumed a normal dark 2Department of Physiology and Biophysics resting current of 25 pA; mouse rod responses in excess of Zilkha Neurogenetic Institute 20 pA are routinely observed in our laboratories. Approxi- USC Keck School of Medicine mately 7% of the current is from Na+/Ca2+ exchange, so we Los Angeles, CA 90089 could directly estimate the Na+ influx in darkness (see Supple- USA mental Data available online). We then divided by 3 to calculate 3Department of Zoology ATP consumption by the Na+/K+ pump, because three Na+ University of Cambridge ions are pumped out of the rod for every ATP. The dependence Downing Street of ATP consumption on light intensity over the physiological Cambridge CB2 3EJ range was evaluated from measurements of mouse rod UK current responses to steady illumination [10] and are shown 4Departments of Physiological Science and Ophthalmology in Figure 1A. ATP utilization falls by an amount equivalent to University of California, Los Angeles 2.3 3 106 ATP s21 per pA decrease in inward current, from Los Angeles, CA 90095-7000 about 5.7 3 107 ATP in darkness to zero at an intensity of about USA 104 Rh* s21, which closes all the cGMP-gated channels. -

Non-Hours and Hours of Darkness Requirements
Non-Hours of Darkness Requirements Hours of Darkness: 340.01 (23) means the period of time from one-half hour after sunset to one-half hour before sunrise and all other times when there is not sufficient natural light to render clearly visible any Flags Under Load: Loads over 100 feet long must have red flags hung beneath the load at person or vehicle upon a highway at a distance of 500 feet. 20 foot intervals from the last axle of the power unit to the first axle of the lasted towed “Oversize Load” Sign: Trans 254.10 (4) (b) & MV2605 #18 unit. Each sign shall state, in black letters on a yellow background, "OVERSIZE LOAD," and may not be less than 7 feet long and 18 inches high. The letters of the sign may not be less Flag Size and Color: Trans 254.10 (3) (d) & MV2605 #17 than 10 inches high with a brush stroke of not less than 1.4 inches. Each flag shall be solid red or orange in color, and not less than 18 inches square. Amber Lighting: Trans 254.10 (2) (a) & MV2605 # 15 Corner Flags: Trans 254.10 (3) & MV2605 #17 Flags Amber flashing light meeting the requirements of Trans 254.10 (2) which is visible to the (a) When a vehicle, load, or vehicle and load is overlength, a single flag shall be fastened front of the power unit, from behind the load, and on each side of the load or power at the extreme rear of the load if the overlength or projecting portion is 2 feet wide unit. -

From Darkness to Sun and Stars
From Darkness to Sun and Stars Lesson 1: From darkness God created light, sun, moon and stars. God created light on the first day of creation. On the fourth day he filled the day with the sun and the night with moon and starts. Infants and toddlers can relate to going to sleep at night and waking in the day. Scripture: Genesis 1:3-5, 14-19 “…God called the light “day,” and the darkness he called “night.”…God made two great lights—the greater light to govern the day and the lesser light to govern the night. He also made the stars.” Teaching Items to Collect Class Schedule (Some are in the Theme Boxes) (45 minutes) Peep tubes (tins that children Welcome Time (15 minutes) look into to view what is On the mat in the soft corner. Time to settle inside): in and free play. Music or singing. o Light o Sun Bible Time and Lesson (20 minutes total) o Moon and Stars At the table Glittery silver star on a stick. Bible Time Lesson: Talk about the fact that God Star stickers made sun, moon and stars. Various toy dolls, animals and Experiment with light and dark with blankets (You will pretend to torches/flashlights. put them to sleep while it is Sing “Twinkle, Twinkle Little Star” or night-dark and wake them “This Little Light of Mine”. Pretend when it is morning-light.) putting babies and animals down to Torches/Flashlights sleep and then waking them up. Craft: (optional) Black & light blue Fairy lights paper for day/night. -

170. Use of Infrared Radiation in the Study of Fish Behavior
USE OF INFRARED RADIATION IN THE STUDY OF FISH BEHAVIOR MarineLIBRARYBiological Laboratory MAY 8- iy56 WOODS HOLE, MASS. SPECIAL SCIENTIFIC REPORT- FISHERIES No. 170 UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE EXPLANATORY NOTE The series embodies results of investigations, usually of restricted scope, intended to aid or direct management or utilization practices and as guides for administrative or legislative action, it is issued in limited quantities for official use of Federal, State or cooperating agencies and in processed form for economy and to avoid delay in publication United States Department of the Interior, Douglas McKay, Secretary Fish and Wildlife Service, John L. Farley, Director USE OF INFRARED RADIATION IN THE STUDY OF FISH BEHAVIOR by Rea E . Duncan Fishery Research Biologist Special Scientific Report- -Fisheries No. 170. Washington, D. C. March 1956 ABSTRACT Infrared radiation can be used to observe the actions of fish in the dark. Experi- ments demonstrated that the behavior of fingerling silver salmon (O^. kisutch ) was not affected by infrared radiation. The infrared filters used did not pass wavelengths visible to an observer, and the actions of the fish were observed through an infrared viewer. The fish were not attracted or repelled by steady radiation, and they did not exhibit a fright reaction to flashing radiation. The orientation pattern in still or flowing water was not affected; there was no indication that the fish could perceive the wavelengths employed. TABLE OF CONTENTS Page INTRODUCTION 1 Problem 1 Water Penetration 1 The Eye and the Spectrum 3 Infrared Radiation and Animals Other Than Fishes 3 MATERIALS 4 EXPERIMENTS ON THE REACTION OF FISH TO INFRARED RADIATION .. -

Control of Rhodopsin Activity in Vision
Control of rhodopsin DENIS A. BAYLOR, MARIE E. BURNS activity in vision Abstract high concentration in the cytoplasm in darkness and that binds to cationic channels in the Although rhodopsin's role in activating the surface membrane, holding them open. phototransduction cascade is well known, the Hydrolysis of cGMP allows the channels to processes that deactivate rhodopsin, and thus close, interrupting an inward current of sodium, the rest of the cascade, are less well calcium and magnesium ions and producing a understood. At least three proteins appear to hyperpolarisation of the membrane. The play a role: rhodopsin kinase, arrestin and hyperpolarisation reduces the rate at which recoverin. Here we review recent physiological neurotransmitter is released from the synaptic studies of the molecular mechanisms of terminal of the rod. rhodopsin deactivation. The approach was to The purpose of this paper is to review recent monitor the light responses of individual work on the important but still poorly mouse rods in which rhodopsin was altered or understood mechanisms that terminate the arrestin was deleted by transgenic techniques. light-evoked catalytic activity of rhodopsin. Removal of rhodopsin's carboxy-terminal These mechanisms, which fix the intensity and residues which contain phosphorylation sites duration of the activation of the transduction implicated in deactivation, prolonged the flash cascade, need to satisfy strong functional response 20-fold and caused it to become constraints. Rhodopsin activity must be highly variable. In rods that did not express arrestin the flash response recovered partially, terminated rapidly so that an absorbed photon but final recovery was slowed over lOO-fold. -

Temporal and Spatial Dependence of Adaptation on Ganglion Cells
Temporal and Spatial Dependence of Adaptation on Ganglion Cells Amin, A.P.1, Moazzezi, R.2, Werblin, F.S.2 1University of California, Berkeley; Department of Integrative Biology 2University of California, Berkeley; Department of Molecular and Cellular Biology Keywords: adaptation, contrast sensitivity, ganglion, inter-stimuli interval, ISI, lateral interneurons, luminosity, MicroElectrode Array (MEA), photoreceptor, rabbit, retina, spatial, temporal ABSTRACT BSJ One of the visual system’s many tasks is to be able to the interval between two circular stimuli (inter-stimuli distinguish objects from the background. The ability interval; ISI) of the same diameter, and by changing the to do this is limited and affected by the relationship diameter over a common ISI, we measured a ganglion between the object (or stimulus) of interest and the cell’s output for one stimulus relative to another background. Adaptation in retinal neurons is the process stimulus. The results show saturation (loss of output to of changing the cell’s response to a stimulus according the 2nd stimulus) of stimuli at lower ISIs. Also, the degree to that stimulus’s background. When the stimulus is of saturation for a given ISI depends on the diameter hard to discern from the background, the retina adapts of the stimulus. These combinations of results illustrate by improving its sensitivity to low contrast. The large the temporal and spatial dependence of adaptation response range maintained by adaptation comes at a on ganglion cells. A larger-diameter stimulus involves cost, however. Adaptation complicates neural coding by multiple neurons surrounding the ganglion cell being making the brain interpret identical stimuli as different recorded so various pathways most likely influence based on differences in background. -

The Eye and Night Vision
Source: http://www.aoa.org/x5352.xml Print This Page The Eye and Night Vision (This article has been adapted from the excellent USAF Special Report, AL-SR-1992-0002, "Night Vision Manual for the Flight Surgeon", written by Robert E. Miller II, Col, USAF, (RET) and Thomas J. Tredici, Col, USAF, (RET)) THE EYE The basic structure of the eye is shown in Figure 1. The anterior portion of the eye is essentially a lens system, made up of the cornea and crystalline lens, whose primary purpose is to focus light onto the retina. The retina contains receptor cells, rods and cones, which, when stimulated by light, send signals to the brain. These signals are subsequently interpreted as vision. Most of the receptors are rods, which are found predominately in the periphery of the retina, whereas the cones are located mostly in the center and near periphery of the retina. Although there are approximately 17 rods for every cone, the cones, concentrated centrally, allow resolution of fine detail and color discrimination. The rods cannot distinguish colors and have poor resolution, but they have a much higher sensitivity to light than the cones. DAY VERSUS NIGHT VISION According to a widely held theory of vision, the rods are responsible for vision under very dim levels of illumination (scotopic vision) and the cones function at higher illumination levels (photopic vision). Photopic vision provides the capability for seeing color and resolving fine detail (20/20 of better), but it functions only in good illumination. Scotopic vision is of poorer quality; it is limited by reduced resolution ( 20/200 or less) and provides the ability to discriminate only between shades of black and white.