Patterns of Mercury Burden in the Seabird Community of Machias Seal Island, New Brunswick
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grand Manan Channel – Southern Part NOAA Chart 13392
BookletChart™ Grand Manan Channel – Southern Part NOAA Chart 13392 A reduced-scale NOAA nautical chart for small boaters When possible, use the full-size NOAA chart for navigation. Published by the 33-foot unmarked rocky patch known as Flowers Rock, 3.9 miles west- northwestward of Machias Seal Island, the channel is free and has a National Oceanic and Atmospheric Administration good depth of water. The tidal current velocity is about 2.5 knots and National Ocean Service follows the general direction of the channel. Daily predictions are given Office of Coast Survey in the Tidal Current Tables under Bay of Fundy Entrance. Off West Quoddy Head, the currents set in and out of Quoddy Narrows, forming www.NauticalCharts.NOAA.gov strong rips. Sailing vessels should not approach West Quoddy Head too 888-990-NOAA closely with a light wind. North Atlantic Right Whales.–The Bay of Fundy is a feeding and nursery What are Nautical Charts? area for endangered North Atlantic right whales (peak season: July through October) and includes the Grand Manan Basin, a whale Nautical charts are a fundamental tool of marine navigation. They show conservation area designated by the Government of Canada. (See North water depths, obstructions, buoys, other aids to navigation, and much Atlantic Right Whales, chapter 3, for more information on right whales more. The information is shown in a way that promotes safe and and recommended measures to avoid collisions with whales.) efficient navigation. Chart carriage is mandatory on the commercial Southwest Head, the southern extremity of Grand Manan Island, is a ships that carry America’s commerce. -

Real Property Issues in the Marine Aquaculture Industry in New Brunswick
REAL PROPERTY ISSUES IN THE MARINE AQUACULTURE INDUSTRY IN NEW BRUNSWICK SUE NICHOLS IAN EDWARDS JIM DOBBIN KATALIN KOMJATHY SUE HANHAM October 2001 TECHNICAL REPORT NO. 208217 REAL PROPERTY ISSUES IN THE MARINE AQUACULTURE INDUSTRY IN NEW BRUNSWICK Sue Nichols Ian Edwards Jim Dobbin Katalin Komjathy Sue Hanham Department of Geodesy and Geomatics Engineering University ofNew Brunswick P.O. Box 4400 Fredericton, N.B. Canada E3B 5A3 October 2001 PREFACE In order to make our extensive series of technical reports more readily available, we have scanned the old master copies and produced electronic versions in Portable Document Format. The quality of the images varies depending on the quality of the originals. The images have not been converted to searchable text. PREFACE This report was prepared under contract for the New Brunswick Department of Fisheries and Aquaculture. The research was carried out in 1997 at the University of New Brunswick, Fredericton, Canada, under the leadership of Professor Sue Nichols. As with any copyrighted material, permission to reprint or quote extensively from this report must be received from the authors. The citation to this work should appear as follows: Nichols, S., I. Edwards, J. Dobbin, K. Komjathy, and S. Hanham (2001). Real Property Issues in the Marine Aquaculture Industry in New Brunswick. Final contract report for the New Brunswick Department of Fisheries and Aquaculture, by the Geographical Engineering Laboratory, Department of Geodesy and Geomatics Engineering Technical Report No. 208, University of New Brunswick, Fredericton, New Brunswick, Canada, 102 pp. Real Property Issues in the Marine Aquaculture Industry in New Brunswick Acknowledgements The authors would like to thank for the Department of Fisheries and Aquaculture for recognising the need for this research and providing the funds. -
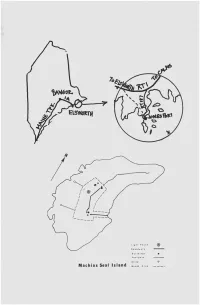
Machias Seal Island Mowad a R I MACHIAS SEAL ISLABD
Foet patK Bltn4 Machias Seal Island Mowad A r i MACHIAS SEAL ISLABD by Paula Butler, Belmont Machias Seal Island— the Contested Island*— has served as a sentinel at the South end of the Bay of Fundy slnce the estahlishment of a lighthouse in 1832. Before that time, this rock (barren except for a highland meadow in summer) vas a marinar's nightmare of sudden fog banks, high seas and hidden rocky shoals. The Harbour Seal, for whioh this island vas probably named, occurs only at a nearby shoal, North Rock. Although today's Journey is quite safe, trips to the island are cancelled vithout notice due to unexpected vind changes or a possible dangerous landing because of ground svells, strong cur- rents and surf. The meadov area of the island contains a variety of plant life: asters, vild parsleys, docks, grasses, sedges, and many other herbs. This island, like many others in the Machias Bay area, vas used for cattle and sheep grazing as vell as Limited farming. Some of these islands vere strategic in the naval maneuvers during the American Revolution. The first naval encounter of that var vas in Machias Bay vhen the villagers of Machias beached the British cutter Margareta. Most birders and photographers are attracted to Machias Seal Island to ob serve Common Puffins (Pratercula árctica) at cióse range. Common Puffins and Razorbills (Alca torda) seem to invite us not only to vatch them but to take delight in posing. Tvo blinds eire provided overlooking the rocky nesting area. The puffins arrive in late April and remain in the vater until they receive a mysterious signal; then, in one large flock, they settle into their nesting sites. -

2 Study Area
CA PDF Page 49 of 1038 Energy East Project Part B: Deterministic Modelling of the Ecological and Volume 24: Ecological and Human Health Risk Human Health Consequences of Marine Oil Spills Assessment for Oil Spills in the Marine Environment Section 2: Study Area 2 STUDY AREA The study area for the analysis of hypothetical crude oil spills for the proposed marine terminal is described in detail in Part A, Section 2 of the EHHRA. This section provides an overview. 2.1 Overview of Canaport Energy East Marine Terminal Operations and Expanded Shipping Operations The proposed marine terminal will be located on the western shore of the Bay of Fundy, southeast of the City of Saint John and southwest of Mispec Point, New Brunswick. The marine terminal is proposed to be located adjacent to the two existing Canaport marine facilities: a single buoy mooring (SBM or mono- buoy) for offloading crude oil to the Irving Canaport facility, and the Canaport LNG terminal for import and now recently licensed for export of liquefied natural gas (Figure 1-1). The marine terminal will include pile-supported trestles and breasting/mooring dolphins for two berths that can accommodate Aframax and Suezmax tankers (Berth 2), as well as VLCC tanker types (Berth 1), with capacities of 113,300 to 348,000 m3 (710,000 to 2.2 million barrels). The two berths will be constructed simultaneously. Oil will be pumped from shore via a trestle approximately 645 m long. Berths 1 and 2 will be interconnected by a trestle approximately 380 m long. The marine terminal is expected to receive approximately 281 tanker calls per year for shipping of crude oil products originating from western Canada. -

Seeing the Light: Report on Staffed Lighthouses in Newfoundland and Labrador and British Columbia
SEEING THE LIGHT: REPORT ON STAFFED LIGHTHOUSES IN NEWFOUNDLAND AND LABRADOR AND BRITISH COLUMBIA Report of the Standing Senate Committee on Fisheries and Oceans The Honourable Fabian Manning, Chair The Honourable Elizabeth Hubley, Deputy Chair October 2011 (first published in December 2010) For more information please contact us by email: [email protected] by phone: (613) 990-0088 toll-free: 1 800 267-7362 by mail: Senate Committee on Fisheries and Oceans The Senate of Canada, Ottawa, Ontario, Canada, K1A 0A4 This report can be downloaded at: http://senate-senat.ca Ce rapport est également disponible en français. MEMBERSHIP The Honourable Fabian Manning, Chair The Honourable Elizabeth Hubley, Deputy Chair and The Honourable Senators: Ethel M. Cochrane Dennis Glen Patterson Rose-Marie Losier-Cool Rose-May Poirier Sandra M. Lovelace Nicholas Vivienne Poy Michael L. MacDonald Nancy Greene Raine Donald H. Oliver Charlie Watt Ex-officio members of the committee: The Honourable Senators James Cowan (or Claudette Tardif) Marjory LeBreton, P.C. (or Claude Carignan) Other Senators who have participated on this study: The Honourable Senators Andreychuk, Chaput, Dallaire, Downe, Marshall, Martin, Murray, P.C., Rompkey, P.C., Runciman, Nancy Ruth, Stewart Olsen and Zimmer. Parliamentary Information and Research Service, Library of Parliament: Claude Emery, Analyst Senate Committees Directorate: Danielle Labonté, Committee Clerk Louise Archambeault, Administrative Assistant ORDER OF REFERENCE Extract from the Journals of the Senate, Sunday, June -

Triassic Basin Stratigraphy at Grand Manan, New Brunswick, Canada J
Document generated on 09/27/2021 4:33 p.m. Atlantic Geology Triassic Basin Stratigraphy at Grand Manan, New Brunswick, Canada J. Gregory. McHone Volume 47, 2011 Article abstract The island of Grand Manan (Canada) in the southwestern Bay of Fundy has the URI: https://id.erudit.org/iderudit/ageo47art06 only exposed strata and basalt of the Grand Manan Basin, a mainly submerged Early Mesozoic riſt basin about 30 km wide by 70 km long. The basin is See table of contents bounded on the southeast by the west-dipping Red Point Fault, which bisects the island, and on the northwest by a submarine border fault marked by the Murr Escarpment, a bathymetric feature that parallels the coast of Maine Publisher(s) (USA). A fault-bounded horst of Ediacaran to Cambrian rocks separates the Grand Manan Basin from the much larger Fundy Basin to the east. The Atlantic Geoscience Society Ashburton Head, Seven Days Work, and Southwest Head members of the end-Triassic Dark Harbour Basalt cover most of western Grand Manan with a ISSN total thickness around 240 m. Up to 12 m of sub-horizontal grey mudstone and fine-grained red sandstone of the Dwellys Cove Formation are exposed along 0843-5561 (print) the western shoreline beneath the basalt. Coarse red arkosic sandstone a few 1718-7885 (digital) metres thick at Miller Pond Road rests on a basement of Late Ediacaran rocks east of the basin. Exposures of the Dwellys Cove and Miller Pond Road Explore this journal formations are at the top and bottom, respectively, of several km (?) of sub-horizontal Late Triassic clastic basin strata, juxtaposed by the eastern border fault. -

New Brunswick Eastern Habitat Joint Venture Five Year Implementation Plan for the North American Waterfowl Management Plan
New Brunswick Eastern Habitat Joint Venture Five Year Implementation Plan for the North American Waterfowl Management Plan New Brunswick Eastern Habitat Joint Venture December 14 2007 Executive Summary The New Brunswick Eastern Habitat Joint Venture (NB-EHJV) was established in 1989 to implement the North American Waterfowl Management Plan (NAWMP) in New Brunswick through a partnership of federal, provincial and regional conservation organizations. The NB-EHJV Five Year Implementation Plan to be known as the Plan is an update of the 1989 – 2004 NB Eastern Habitat Joint Venture Implementation Plan. The Plan outlines the current status of wetland habitats and waterfowl populations in New Brunswick and presents waterfowl population goals and habitat conservation targets for the next five years. The Plan is a blueprint for the conservation of important breeding, staging and wintering waterfowl habitat in New Brunswick. It describes the habitat conservation program the NB- EHJV partnership will employ to achieve the waterfowl population objectives, the methods for program assessment and the resource needs. Though this Plan may benefit other bird species, it does not explicitly address the objectives for the other bird Pillars of the North American Bird Conservation Initiative (NABCI). The Plan will address 15% (1,105 IBP) of the 7,600 IBP population deficit for dabbling ducks (American black duck, Mallard, green-winged teal, ring-necked duck) in NB through the enhancement or restoration of 1,380 hectares of wetlands and the active management of 13,901 hectares of wetlands to optimum waterfowl brood rearing conditions. A nest box program will be used to increase wood duck and common goldeneye. -

Machias Seal Island Quartz Monzodiorite: the Southernmost Rocks in New Brunswick
72 AGS Abstracts – 2006 Annual General Meeting Machias Seal Island quartz monzodiorite: the southernmost rocks in New Brunswick Sandra M. Barr1, James K. Mortensen2, and Heather E. Wolczanski1, 1. Department of Geology, Acadia University, Wolfville, NS, B4P 2R6 <[email protected]> ¶ 2. Earth & Ocean Sciences, University of British Columbia, Vancouver, BC, V6T 1Z4 Machias Seal Island is located at the mouth of the Bay of Fundy, about 20 km south of Grand Manan Island and 20 km east of Machias, Maine. The island has an area of about 0.04 km2, and is barren except for a lighthouse and dwell- ings maintained by the government of Canada. It has the last manned lighthouse in the Maritime Provinces, and is well known among bird-watchers as a nesting site for puffi ns, auks, and other seabirds. Geologically, Machias Seal Island is important because of its location in an area through which it is diffi cult to trace terranes from the mainland of Nova Scotia and New Brunswick into the New England states. Studies in Grand Manan Island have not entirely resolved the problem, as rocks there most resemble those of the New River terrane of southern New Brunswick, suggesting that the Kingston, Brookville, Caldonia (Avalon), and Meguma terranes all lie outboard of Grand Manan Island. Machias Seal Island consists entirely of grey, locally pink, fi ne- to medium-grained weakly foliated quartz monzodiorite, gradational to granodiorite. Small ovoid metadioritic xeno- Atlantic Geology, 2006, Volume 42, Number 1 Copyright © 2015 Atlantic Geology Atlantic Geology 73 liths, generally less than 20 cm in maximum dimension, are abundant and consist of fi ne-grained granoblastic plagioclase, orthopyroxene, clinopyroxene, amphibole, and biotite. -

Science Review 1988 &
Science Review 1988 & ‘89 (This Page Blank in the Original) Research 1988 and ‘89 in review S. B. MacPhee, D. I. Ross, and H. B. Nicholls Society, replacing Dr. David Aiken; = Dr. Trevor Platt (DFO) was the 1988 re- cipient of the G. Evelyn Hutchinson Medal of the American Society of Limnology and Oceanography, the highest award given by the society; = Kate Moran (DEMR) was appointed Chairperson of the Shipboard Measure- ments Panel of the international Ocean Drilling Project; and = Dr. A.R. Longhurst (DFO) was elected a Fellow of the Royal Society of Canada. D. I. Ross. S. B. MacPhee, and H. B. Nicholls Huntsman Award: The A. G. Hunts- man Award for excellence in the marine The years 1988 and ‘89 were interest- Hydrography Branch, Mr. Paul Bellemare sciences is awarded annually. It is admin- ing and fruitful ones for the research and was appointed Regional Director of istered by a private foundation based at survey programs carried out at the Bed- Hydrography in May 1988. Subsequently, BIO. ford Institute of Oceanography, the Hali- Mr. Reginald Lewis was appointed Re- The following two Huntsman Awards fax Fisheries Research Laboratory, and gional Field Superintendent. were presented during the period covered the St. Andrews Biological Station. In the The following senior staff changes by this Review. following paragraphs, information is pro- were made in the Atlantic vided on a number of the significant Geoscience Centre (AGC) of events that occurred during those years at DEMR. In August 1988, Dr. the laboratories of the Department of Fish- David Ross was appointed Direc- eries and Oceans (DFO) and at those of the tor of AGC, succeeding Dr. -

70 Dominion Marine.
70 Dominion Marine. ... L- ...... HI are placed at the following places: Mirima- take them off, as soon as convenient, and chi, Caraquette, Richibucto, Shediac, St. supply their place with cattle, which may be George, Bathurst, St. Andrews, Shippegan, available for food for shipwrecked crews. Buctouche, Campbelton, Cocaigne, Bay Three wrecks took place at this island during Verte and St. John. The cost of this service last year. for the fiscal year was $3,711. Previous to The sum of $5,000 has been voted by Par Confederation it was defrayed by tonnage liament for constructing a Light House on duty on shipping; but now, no dues are ex Sable Island. It is proposed to place, in the acted. spring, a powerful Dioptric light, to be visi A Signal station is maintained at Partridge ble thirty miles. Sand bars run out a long Island, entrance of St. John harbour, for distance from the island, and it is important communicating the arrival of vessels. to give vessels timely warning. It is also The Light House service in New Bruns proposed to place powerful steam Fog Whis wick is managed by an agent resident in St. tles on this Island. John, under the direction of the Department. Thero are now thirty-five lights in operation OIL SUPPLIES. in New Brunswick, of which nineteen are sea lights. The quantity of Oil required for the Light The new light at Jourimain is visible six House service of the Dominion for the last teen miles. The new Light House on Ma- financial year, was 32,000 gallons, which was chias Seal Island, together with the repairs supplied in bond at 22£ cents per gallon; the to the old light house tower at this station, kind used is the best quality of refined petro and the application of new lights are impor leum oil, of Canadian manufacture. -

Lighthouses in Manitoba Petitioned to Be Considered for Heritage Designation Under the Heritage Lighthouse Protection Act
Heritage Lighthouse Programme des Program phares patrimoniaux parcscanada.gc.ca parkscanada.gc.ca The Minister responsible for Parks Canada will consider all lighthouses for which a petition meeting the requirements of the Act was received and determine which should be designated as heritage lighthouses on or before 29 May 2015, taking into account the advice of an advisory committee and the established criteria. To learn more about processes related to the evaluation and designation of petitioned lighthouses, please visit our website at www.parkscanada.gc.ca/lighthouses. Lighthouses in British Columbia petitioned to be considered for heritage designation under the Heritage Lighthouse Protection Act Province Lighthouse DFRP # BC Active Pass 17248 BC Addenbroke Island 67677 BC Amphitrite Point 17923 BC Ballenas Islands 17675 BC Boat Bluff 67678 BC Bonilla Island 19482 BC Cape Beale 17809 BC Cape Mudge 18225 BC Cape Scott 19007 BC Carmanah Point 17533 BC Chatham Point 18090 BC Chrome Island Range 18001 BC Discovery Island 17425 BC Dryad Point 67679 BC East Point (Saturna Island) 17296 BC Egg Island 67680 BC Entrance Island 17611 BC Estevan Point 17813 BC Fisgard 17454 BC Green Island (BC) 67681 BC Ivory Island 67682 BC Langara Point 19401 BC Lennard Island 17812 BC Lucy Islands 84377 Heritage Lighthouse Program, Parks Canada Page 1 of 2 25 Eddy (25-5-P), Gatineau QC K1A 0M5 Telephone 819-934-9096 Generated: 31 July 2012 Facsimile 819-953-4139 [email protected] | www.parkscanada.gc.ca/lighthouses Heritage Lighthouse Programme des Program phares patrimoniaux parcscanada.gc.ca parkscanada.gc.ca The Minister responsible for Parks Canada will consider all lighthouses for which a petition meeting the requirements of the Act was received and determine which should be designated as heritage lighthouses on or before 29 May 2015, taking into account the advice of an advisory committee and the established criteria. -

History Islands and Isletes in the Bay of Fundy
V r iiis'ronv OF IN THE BAY OF XI) Y, Charlotte County, New Bmuswick: HjOM THE IE EARLIEST SETTLEMENT TO THE t>KEbf.NT time; ixoLimixo Sketches of Shipwrecks and Other Events of Exciting Interest BY J. G. LOEIMER, Esq f>N. B. 'iUX I EU AT THE OFFICE OF THE SAINT CKOIX COURIEE 1<S76 » — t- I CONTESTS. CHAPTER I. INTRODUCTORY. The Bay- Its Peculiarities—Rivers—Capes—Cape Blomidon—Basin of Minas—Tides—Fogs—Bay de Verte —Wellington Dyke—Counties Washed, &c. Page 5. CHAPTER II. GRAND MANAN, Early History— Seal Cove—Outer Islands—Grand Harbour—Wood- ward's Cove—White Head—Centrevilie —North Head—Eel Brook Long Island—Other Islets—Shipwrecks—Minerals, <§&. Page 11. CHAPTER III. MACHIAS SEAL ISLAND. Description—The Connollys—Lights—Shipwrecks—Fish and Fowl, &c. Page 71. CHAPTER IV. INDIAN ISLAND Early History—James Ohaflfey—Indian Belies—Le Fontaine—Smug- gling—Fight for Tar-^Capture of Schooner—John Doyle's Song —Fenians and the British Flag—Stores Burnt—Island Fleet—Customs —Indians' Burying Ground, &c. Page 73. CHAPTER V. DEER ISLAND. Early History—Peculiarity of Road System—Fish less Lake—Indian Relics—Coves—Churches—Schools — Temperaace—Whirlpools—Loss . — 4 Contents. of Life—Boat swallowed up in Whirlpools— Pious Singing Saves a Boatman—Lobster Factory, &c. Page 89. CHAPTER VL C AMPOBELLO Early History— Surveying Steamer Columbia—Minerals— Harbours Lighthouse—Churches— Schools— VVelchpool —Wilson's Beach—Admi- £ ral Owen — Captain Robinson-Owen— 'ish Fairs—Boat Racing—Vete- ran Mail Carrier—Central Road-— Harbour de Lute, &c. Page 97. CHAPTER VIL RECAPITULATORY AND CONCLUSIVE, Remarks -The Bay— The " Bore "—Lives Lost —Five Lights—Salt- Water Triangle—Militia Training - G teat Hole Through a Cliff— Wash- ington and Wellington—Minerals— Beautiful Specimens—New Weir —Porpoise Shooting—Fertility—Houses and Stores —Pedlars—Post- Offices—Mail-Vessels—Schooneis—Boats and Steamer—Singular Fruit —General Review—Closing Remarks.