Active Conventional Treatment and Three Different Biological Treatments in Early Rheumatoid Arthritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Xolair/Omalizumab
Therapeutic/Humanized Antibodies ELISA Kits ‘Humanized antibodies” are ‘Animal Antibodies’ that have been modified by recombinant DNA-technology to reduce the overall content of the animal- portion of immunoglobulin so as to increase acceptance by humans or minimize ‘rejection’. By analogy, if the hands of a mouse is actually responsible for grabbing things then ‘humanized-mouse’ will only contain the ‘mouse hands’ and the rest of the body will be human. Since the size of the IgGs is similar in mouse and human, the size of the native mouse IgG and the ‘humanized IgG’ does not significantly change. Antigen recognition property of an antibody actually resides in small portion of the IgG molecule called the ‘antigen binding site or Fab’. Therefore, humanized antibodies contain the minimal portion from the Fab or the epitopes necessary for antigen binding. The process of "humanization of antibodies" is usually applied to animal (mouse) derived monoclonal antibodies for therapeutic use in humans (for example, antibodies developed as anti-cancer drugs). Xolair (Anti-IgE) is an example of humanized IgG. The portion of the mouse IgG that remains in the ‘humanized IgG’ may be recognized as foreign by humans and may result into the generation of “Human Anti-Drug Antibodies (HADA). The presence of anti-Drug antibody (e.g., Human Anti-Rituximab IgG) that may limit the long-term usage the humanized antibody (Rituximab). Not all monoclonal antibodies designed for human therapeutic use need be humanized since many therapies are short-term. The International Nonproprietary Names of humanized antibodies end in “-zumab”, as in “omalizumab”. Humanized antibodies are distinct from chimeric or fusion antibodies. -

Australian Public Assessment Report for Abatacept (Rch)
Australian Public Assessment Report for Abatacept (rch) Proprietary Product Name: Orencia Sponsor: Bristol-Myers Squibb Australia Pty Ltd June 2011 About the Therapeutic Goods Administration (TGA) · The TGA is a division of the Australian Government Department of Health and Ageing, and is responsible for regulating medicines and medical devices. · TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance), when necessary. · The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. · The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. · To report a problem with a medicine or medical device, please see the information on the TGA website. About AusPARs · An Australian Public Assessment Record (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. · AusPARs are prepared and published by the TGA. · An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations, and extensions of indications. · An AusPAR is a static document, in that it will provide information that relates to a submission at a particular point in time. · A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0209462 A1 Bilotti Et Al
US 20170209462A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0209462 A1 Bilotti et al. (43) Pub. Date: Jul. 27, 2017 (54) BTK INHIBITOR COMBINATIONS FOR Publication Classification TREATING MULTIPLE MYELOMA (51) Int. Cl. (71) Applicant: Pharmacyclics LLC, Sunnyvale, CA A 6LX 3/573 (2006.01) A69/20 (2006.01) (US) A6IR 9/00 (2006.01) (72) Inventors: Elizabeth Bilotti, Sunnyvale, CA (US); A69/48 (2006.01) Thorsten Graef, Los Altos Hills, CA A 6LX 3/59 (2006.01) (US) A63L/454 (2006.01) (52) U.S. Cl. CPC .......... A61 K3I/573 (2013.01); A61K 3 1/519 (21) Appl. No.: 15/252,385 (2013.01); A61 K3I/454 (2013.01); A61 K 9/0053 (2013.01); A61K 9/48 (2013.01); A61 K (22) Filed: Aug. 31, 2016 9/20 (2013.01) (57) ABSTRACT Disclosed herein are pharmaceutical combinations, dosing Related U.S. Application Data regimen, and methods of administering a combination of a (60) Provisional application No. 62/212.518, filed on Aug. BTK inhibitor (e.g., ibrutinib), an immunomodulatory agent, 31, 2015. and a steroid for the treatment of a hematologic malignancy. US 2017/0209462 A1 Jul. 27, 2017 BTK INHIBITOR COMBINATIONS FOR Subject in need thereof comprising administering pomalido TREATING MULTIPLE MYELOMA mide, ibrutinib, and dexamethasone, wherein pomalido mide, ibrutinib, and dexamethasone are administered con CROSS-REFERENCE TO RELATED currently, simulataneously, and/or co-administered. APPLICATION 0008. In some aspects, provided herein is a method of treating a hematologic malignancy in a subject in need 0001. This application claims the benefit of U.S. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Download (PDF)
Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Information for the public Published: TBC nice.org.uk What has NICE said? Abatacept (Orencia), adalimumab (Humira), etanercept (Enbrel) and tocilizumab (RoActemra) are recommended as possible treatments for people with polyarticular juvenile idiopathic arthritis. Adalimumab and etanercept are recommended as possible treatments for people with enthesitis- related juvenile idiopathic arthritis. Etanercept is recommended as a possible treatment for people with psoriatic juvenile idiopathic arthritis. What does this mean? If your (or your child's) doctor thinks that abatacept, adalimumab, etanercept or tocilizumab are the right treatment, you (or your child) should be able to have the treatment on the NHS. Abatacept, adalimumab, etanercept and tocilizumab should be available on the NHS within 3 months of the guidance being issued. If you (or your child) are not eligible for treatment as described above, you (or your child) should be able to continue taking abatacept, adalimumab, etanercept or tocilizumab until you and your (or your child's) doctor decide it is the right time to stop. © NICE TBC. All rights reserved. Page 1 of 3 Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Why has NICE said this? NICE looks at how well treatments work in relation to how much they cost compared with other treatments available on the NHS. Abatacept, adalimumab, etanercept and tocilizumab were recommended because the benefits to patients justify their cost. The condition and the treatments Arthritis causes pain and inflammation in joints. Juvenile idiopathic arthritis is a type of arthritis that starts in people younger than 16 years (juvenile). -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -

Re-Treatment with Abatacept Plus Methotrexate for Disease Flare After Complete Treatment Withdrawal in Patients with Early Rheum
Rheumatoid arthritis RMD Open: first published as 10.1136/rmdopen-2018-000840 on 8 February 2019. Downloaded from ORIGINAL ARTICLE Re-treatment with abatacept plus methotrexate for disease flare after complete treatment withdrawal in patients with early rheumatoid arthritis: 2-year results from the AVERT study Paul Emery,1,2 Gerd R Burmester,3 Vivian P Bykerk,4 Bernard G Combe,5 Daniel E Furst,6 Michael A Maldonado,7 Tom WJ Huizinga8 To cite: Emery P, Burmester GR, ABSTRACT Bykerk VP, et al. Re- Objectives To complete reporting of outcomes after total Key messages treatment with abatacept withdrawal of all rheumatoid arthritis (RA) therapy and re- plus methotrexate for disease treatment after flare inA ssessing Very Early Rheumatoid What is already known about this subject? flare after complete treatment arthritis Treatment study (NCT01142726). ► Abatacept in combination with methotrexate induc- withdrawal in patients with es remission more often than methotrexate alone early rheumatoid arthritis: Methods Patients with early RA were initially randomised to double-blind, weekly subcutaneous abatacept plus after 12 months of treatment, with a greater propor- 2-year results from the tion of patients with rheumatoid arthritis (RA) main- AVERT study. methotrexate, or abatacept or methotrexate monotherapy. RMD Open taining remission over 6 months following treatment 2019;5:e000840. doi:10.1136/ At month 12, patients with Disease Activity Score (DAS)28 rmdopen-2018-000840 C reactive protein (CRP) <3.2 had all RA treatments rapidly withdrawal. withdrawn and were observed for ≤12 months or until What does this study add? flare.A fter ≥3 months’ withdrawal, patients with protocol- ► Prepublication history and ► These final results of theA ssessing Very Early additional material for this defined RA flare received open-label abatacept plus Rheumatoid arthritis Treatment (AVERT) study paper are available in online. -
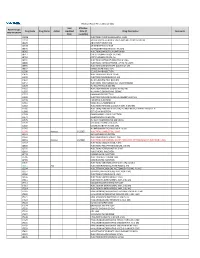
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Cimzia (Certolizumab Pegol) AHM
Cimzia (Certolizumab Pegol) AHM Clinical Indications • Cimzia (Certolizumab Pegol) is considered medically necessary for adult members 18 years of age or older with moderately-to-severely active disease when ALL of the following conditions are met o Moderately-to-severely active Crohn's disease as manifested by 1 or more of the following . Diarrhea . Abdominal pain . Bleeding . Weight loss . Perianal disease . Internal fistulae . Intestinal obstruction . Megacolon . Extra-intestinal manifestations: arthritis or spondylitis o Crohn's disease has remained active despite treatment with 1 or more of the following . Corticosteroids . 6-mercaptopurine/azathioprine • Certollizumab pegol (see note) is considered medically necessary for persons with active psoriatic arthritis who meet criteria in Psoriasis and Psoriatic Arthritis: Biological Therapies. • Cimzia, (Certolizumab Pegol), alone or in combination with methotrexate (MTX), is considered medically necessary for the treatment of adult members 18 years of age or older with moderately-to-severely active rheumatoid arthritis (RA). • Cimzia (Certolizumab pegol is considered medically necessary for reducing signs and symptoms of members with active ankylosing spondylitis who have an inadequate response to 2 or more NSAIDs. • Cimzia (Certolizumab Pegol) is considered investigational for all other indications (e.g.,ocular inflammation/uveitis; not an all-inclusive list) because its effectiveness for indications other than the ones listed above has not been established. Notes • There are several brands of targeted immune modulators on the market. There is a lack of reliable evidence that any one brand of targeted immune modulator is superior to other brands for medically necessary indications. Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab), Simponi (golimumab), Simponi Aria (golimumab intravneous), and Stelara (ustekinumab) brands of targeted immune modulators ("least cost brands of targeted immune modulators") are less costly to the plan. -

Anti-TNF-Α (Certolizumab), Humanized Antibody
FOR RESEARCH USE ONLY! rev 06/21 Anti-TNF-α (Certolizumab), Humanized Antibody CATALOG NO.: A2142-100 (100 µg) BACKGROUND DESCRIPTION: The research-grade biosimilar is a humanized TNF-α monoclonal antibody. The original monoclonal antibody (Certolizumab pegol (CZP)) consists of polyethylene glycol conjugated to the antigen-binding fragment of the humanized monoclonal antibody. The antibody targets and neutralizes soluble and membrane forms of tumor necrosis factor-alpha (TNF-α) and inhibits TNF-α mediated signaling in vitro. Unlike other TNF inhibitors, this antibody does not contain the Fc region. As a result, decreased Fc-mediated effects such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are observed. The original monoclonal antibody received FDA approval to treat moderate to severe rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, and psoriatic arthritis. TNFSF2, TNF-alpha, TNFA, TNF-α, TNFα ALTERNATE NAMES: ANTIBODY TYPE: Monoclonal HOST/ISOTYPE: Recombinant / Fab-IgG1-kappa SOURCE: CHO cells IMMUNOGEN: Human TNF-α FORM: Liquid FORMULATION: In PBS, pH 7.5 SPECIES REACTIVITY: Human STORAGE CONDITIONS: Store at -80 ºC. Avoid repeated freeze-thaw cycles This information is only intended as a guide. The optimal dilutions must be determined by the user RELATED PRODUCTS: Anti-TNF-α (Adalimumab), humanized Antibody (Cat. No. A1048) Anti-TNF alpha (Infliximab), Human IgG1 Antibody (Cat. No. A1097) Anti-TNF alpha (Humicade), Human IgG4 Antibody (Cat. No. A1093) Anti-EGFR (Cetuximab), Chimeric Antibody (Cat. No. A1047) Anti-EGFR (Matuzumab), Human IgG1 Antibody (Cat. No. A1090) FOR RESEARCH USE ONLY! Not to be used on humans. 155 S. Milpitas Blvd., Milpitas, CA 95035 USA | T: (408)493-1800 F: (408)493-1801 | www.biovision.com | [email protected] . -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH])