Pigeon Lake Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amateur Photo Contest Winner Fall Scenery & Nature Alie Forth “Cattle
Amateur Photo Contest 2017 1st Place Winner Phyllis Cleland “Autumn Harvest” Amateur Photo Contest 2017 2nd Place Winner Lee Fredeen Kohlert “Water Lily” Amateur Photo Contest 2017 Adam & Sandra Goble “Splash” Amateur Photo Contest 2017 Adam & Sandra Goble “Reflections” Amateur Photo Contest 2017 Mary Whitefish “Lost & Forgotten” Amateur Photo Contest 2017 Mary Whitefish “Fiery Sky” Amateur Photo Contest 2017 Mary Whitefish “Bird on a Wire” Amateur Photo Contest 2017 Mary Whitefish “Bambi” Amateur Photo Contest 2017 Mary Whitefish “Winter’s Tundra” Amateur Photo Contest 2017 Brian Rabel “Solitude” Amateur Photo Contest 2017 Brian Rabel “Sunrise on the Lake” Amateur Photo Contest 2017 Brian Rabel “Red Sky in Morning” Amateur Photo Contest 2017 Brian Rabel “Sunset & Second Cut” Amateur Photo Contest 2017 Brian Rabel “Bluebird Skies” Amateur Photo Contest 2017 Tracy Pepin “Love Alberta Beef” Amateur Photo Contest 2017 Tracy Pepin “Fields of Golds” Amateur Photo Contest 2017 Tracy Pepin “Creekside Retreat” Amateur Photo Contest 2017 Tracy Pepin “Homesteads” Amateur Photo Contest 2017 Tracy Pepin “Rainy Day on the Lake” Amateur Photo Contest 2017 Katelyn Van Haren “Bison in the Moonlight” Amateur Photo Contest 2017 Deborah Bailer “Twin Lakes” Amateur Photo Contest 2017 Deborah Bailer “Twin Lakes” Amateur Photo Contest 2017 Deborah Bailer “Twin Lakes” Amateur Photo Contest 2017 Deborah Bailer “Twin Lakes” Amateur Photo Contest 2017 Meagan Lacoste “Black Capped Chickadee” Amateur Photo Contest 2017 Meagan Lacoste “Mid Summer Blooms” Amateur -
Tourist Guide
TOURIST GUIDE 55 AVENUE WWW.52 AVENUEWETASKIWIN.CA Discover Wetaskiwin Wetaskiwin is a City with a growing population of 12,621 and over 700 businesses; the City offers all urban amenities with the charm of a small town. Whether you know us as a city where “Cars cost less” or home to the Reynolds-Alberta Museum, one thing is for sure, Wetaskiwin welcomes you to an adventure. Take in the Rawhide Rodeo or dance to the music at the Loonstock Music Festival. Visit the Wetaskiwin and District Heritage Museum, the Reynolds- Alberta Museum and Canada’s Aviation Hall of Fame. Enjoy a show at the Manluk Performing Arts Theatre. Feeling adventurous? Take a rare flight in the open cockpit of a Biplane. Looking for family fun? Surf the Board Rider at the Manluk Aquatic Centre. The Edmonton International Raceway, located in Wetaskiwin, hosts the NASCAR 300 lap race. Whatever your pleasure - there is an experience for everyone in one of Alberta’s oldest cities. Visit our website for local events happening in the community, www.wetaskiwin.ca. MUSEUMS 4 Reynolds-Alberta Museum 6 Canada’s Aviation Hall of Fame 8 Wetaskiwin & District Heritage Museum 10 Alberta Central Railway Museum 12 Historic City Hall Tours 14 Wetaskiwin Archives 14 HISTORICAL POINTS OF INTEREST 16 LEISURE & ATTRACTIONS 22 MAP OF WETASKIWIN 28 ACCOMODATIONS 38 RESTAURANTS 42 EXCITING EXCURSIONS 46 VISITORS INFORMATION 48 INDEX 3 MUSEUMS 50 STREET 50 Wetaskiwin is proud to boast of our museums such as the international award-winning Reynolds-Alberta Museum, Canada’s Aviation Hall of Fame, the Wetaskiwin and District Heritage Museum, and the Alberta Central Railway Museum. -

North Pigeon Lake Area Structure Plan
Schedule “A” of Bylaw No. 19 -10, As Adopted – October 5, 2010 As Amended by Bylaw No. 19 -11, May 3, 2011 North Pigeon Lake Area Structure Plan Suite 101 - 1101 5th Street Nisku, AB T9E 2X3 www.leduc-county.com Phone: 780-955-3555 Acknowledgements Contributors: Tom Schwerdtfeger, B.U.R.Pl. Planning Vinod K. Bhardwaj, P. Eng., MCIP Planning Gregory F. Wilkes, MCIP Planning Harry S. Zuzak, P. Eng, Planning & Storm Water Management Challenger Engineering Municipal Engineering Bunt & Associates Transportation Bruce Thompson & Associates Environmental Assessment Omni-McCann Consultants Geotechnical & Hydrogeological Douglas C. Penney, P.Ag. Agricultural Assessment POPULUS Community Planning Inc. Public Engagement Mindsprings Inc. Public Engagement Amanda LeNeve Plan Graphic Design Table of Contents PART A - BACKGROUND 1.0 Introduction 7 1.1 The Plan Intent 8 1.2 Plan Area 9 1.3 Legal Framework 10 2.0 The Planning Process 11 2.1 Public Engagement 11 3.0 Watershed Setting & History 14 4.0 Policy Context 16 4.1 Provincial Context 16 4.2 Regional 17 4.3 County Context 18 4.4 Watershed Planning Reports and Tools 21 5.0 Existing Conditions Analysis 23 5.1 Existing Districting 23 5.2 Natural Environment 25 5.2.1 Ecological Setting 5.2.2 Geology and Soils 5.2.3 Surface Water 5.2.4 Groundwater 5.2.5 Natural Setting 5.2.6 Wildlife and Wildlife Habitat 5.2.7 Fish and Aquatic Systems 5.2.8 Environmental Reserves, Parks and Trails 5.3 Transportation 29 5.4 Geotechgnical and Hydrogeological 30 5.5 Agriculture 31 6.0 Constraints 32 PART B - THE PLAN 7.0 -

PIGEON LAKE, ALBERTA …A Brief History
PIGEON LAKE, ALBERTA …a brief history Pigeon Lake is one of the largest and most extensively used recreational waters in Alberta. The lake covers an area of 96.7 km2 (37.3 sq. mi), and has a maximum depth of 9.1 m (30 ft.) It is an early tributary of the Battle River, connected through the Pigeon Lake Creek with no large water inflows. It is served by hundreds of fresh water streams and artesian wells, with levels highly dependent on snow and rain conditions. The water freezes over in November of each year and over the past half century has thawed to open water as early as April 16 in 2016 and as late as May 28th in 2013. Historical records detail a large artesian well on the northeast corner of the lake used for fresh drinking water by Nakoda tribes and the Algonquin Cree who travelled the region as early as 1725. Anthony Henday, one of the first of the British explorers, travelled the area as an emissary for the Hudson Bay Company in 1754 when the lake was called “hmi-hmoo” by the Cree Indians. The name in English meant "Woodpecker Lake." In 1858 the name was changed to Pigeon Lake in recognition of Passenger Pigeons, considered one of the prettiest doves in the world. They were said to have numbered in the millions and unfortunately were hunted to extinction. In the mid-19th century Pigeon Lake became a gathering place for First Nations people from numerous tribes and therefore a desirable spot for the location of both a Hudson Bay Company Trading Post and a Christian Mission. -

Pigeon Lake Watershed Management Plan
Pigeon Lake Watershed Management Plan This management plan has been adopted by the municipalities listed below, whose councils have each passed the following resolution: Council, having read and considered the Pigeon Lake Management Plan, resolves as follows: 1. to refer proposed major developments within [the municipality] to other municipalities as set out in the plan, 2. to consider the effect on the lake as a whole, and on other municipalities around the lake, before approving any development in the Pigeon Lake watershed, and 3. to use the policies set out in the Management Plan as a guide when making any decision affecting the Pigeon Lake watershed. Municipality Date of adoption Leduc County 11 January 2000 County of Wetaskiwin 08 February 2000 SV of Argentia Beach 29 February 2000 SV of Crystal Springs 15 April 2000 SV of Grandview 22 March 2000 SV of Golden Days 14 March 2000 SV of Itaska Beach 16 March 2000 SV of Ma-Me-O Beach 18 April 2000 SV of Norris Beach 14 March 2000 SV of Poplar Bay 20 April 2000 SV of Silver Beach 04 May 2000 SV of Sundance Beach 23 March 2000 1 PART ONE: TECHNICAL BACKGROUND In the spring of 1997, the two counties and ten summer villages bordering Pigeon Lake, organized as the Association of Pigeon Lake Municipalities (APLM), agreed to fund a study of lake water quality. The purpose was to find out if increasing onshore development had resulted in changes to water quality since the previous 1983 study by Hardy Associates, and how development in the drainage basin should be handled to preserve the recreational value of the lake. -

Pigeon Lake Wilderness Unit Management Plan
De artment of Environmental Conservation Division of Lands and Forests Pigeon Lake Wilderness Area Unit Management Plan October 1992 · New York State Department of Environmental Conservation MARIO M. CUOMO, Governor THOMAS C. JORLING, Commissioner PIGEON LAKE WILDERNESS AREA unit Management Plan October 1992 MEMORANDUM FROM THOMAS C. JORLING, Commissioner New York State Department of Environmental Conservation NOV 2 3 1992 TO: The Record ./", FROM: Thomas c. Jorlt9~ SUBJECT: Unit Management Plan Pigeon Lake Wilderness DATE: The Unit Management Plan for the Pigeon Lake Wilderness has been completed. The Plan is consistent with the guidelines and criteria of the Adirondack Park State Land Master Plan, the State constitution, Environmental Conservation Law, and Department rules, regulations and policies. The Plan includes management objectives for a five-year period and is hereby approved and adopted. cc: L. Marsh PIGBOH LAKB WILDBRHESS AREA "The Pigeon Lake Wilderness Area, with its numerous sparkling lakes, the absence of roads, the divide between numerous water- sheds, is an isolated, little top-of-the-world atmosphere, a haven of great variety that does not offend the senses. There is added a few woodpeckers for noise so the stillness is bearable." S. E. Coutant TABLE OF COllTEHTS I • Introduction . 1 A. Area Description . • • . • . • . 1 B. History . 2 II. Resource Inventory Overview . 4 A. Natural Resources . 4 1. 4 a. Geology . 4 b. 4 c. Terrain . 6 d. Climate . 6 e. Water . 7 f. Wetlands . 8 2. Biological . 9 a. Vegetation . 9 b. Wildlife . •............................................. 11 c. Fisheries . 19 3. Visual . 28 4. Areas and/or Historical Areas .........•..•......... 29 5. Wilderness . -
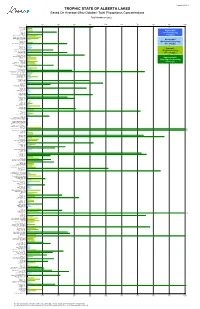
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Mercury in Fish 2009-2013
2016 Mercury in Fish In Alberta Water Bodies 2009–2013 For more information on Fish Consumption Advisories Contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-0-7785- 8283-0 (Report) ISBN: 978-0-7785- 8284-7 (PDF) 2016 Government of Alberta Alberta Health, Health Protection Branch Mercury in Fish in Alberta Water Bodies 2009 – 2013 February 2016 Executive Summary Mercury enters the environment through various natural processes and human activities. Methylmercury is transformed from inorganic forms of mercury via methylation by micro-organisms in natural waters, and can accumulate in some fish. Humans are exposed to very low levels of mercury directly from the air, water and food. Fish consumers may be exposed to relatively higher levels of methylmercury by eating mercury-containing fish from local rivers and lakes. Methylmercury can accumulate in the human body over time. Because methylmercury is a known neurotoxin, it is necessary to limit human exposure. From 2009 to 2013, the Departments of Environment and Parks (AEP) and Health (AH) initiated a survey of mercury levels in fish in selected water bodies in Alberta. These water bodies are extensively accessed by the public for recreational activities. This report deals with (1) concentrations of total mercury levels in various fish species collected from the water bodies in Alberta, (2) estimated exposures, (3) fish consumption limits, (4) fish consumption advisories, and (5) health benefits of fish consumption. The results indicate that: 1. Concentrations of total mercury in fish in the water bodies in Alberta were within the ranges for the same fish species from other water bodies elsewhere in Canada and the United States. -

Cosmetic Lawn Fertilizer Brochure.Pdf
Natural vegetation SUSTAINABLE AGRICULTURE versus turf grass Sustainable Agriculture Turf grass lawns should be Leduc County and the County of Wetaskiwin avoided on waterfront properties No. 10’s shared full-time Sustainable Agriculture Managing your as much as possible, as they are not program manager promotes the awareness and as effective at controlling erosion and adoption of beneficial management practices surface runoff when compared to natural in assisting landowners in becoming educated waterfront lakeshore vegetation. stewards of our land and water resources. It is important that landowners understand the lawn care requirements of waterfront Leduc County 101, 1105 - 5 Street properties. Nisku, AB | T9E 2X3 Landowners are urged to eliminate the use of www.leduc-county.com cosmetic fertilizers, which are fertilizers used on residential properties to promote lawn Kim Barkwell, Program Manager and plant growth. Eliminating the use of all Phone: 780-387-6182 cosmetic fertilizers is ideal, however, if you Email: [email protected] must use fertilizer, organic fertilizers (manure, compost, etc.) are preferred. Leaving grass clippings on the lawn will supply nutrients in place of fertilizers. Landowners should expect to tolerate vegetation diversity on their property and choose drought and disease-tolerant varieties when performing landscaping outside of the County of Wetaskiwin No. 10 buffer zone. 243019A Highway 13 Wetaskiwin, AB | T9A 2G5 Resources www.county.wetaskiwin.ab.ca Alberta Lake Management Society | www.alms.ca Kim Barkwell, Program Manager Cows and Fish | www.cowsandfish.org Phone: 780-387-6182 Living by Water | www.naturealberta.ca Email: [email protected] Pigeon Lake Watershed Association | www.plwa.ca Wizard Lake Watershed and Lake Stewardship Association | www.wizardlake.ca County of Wetaskiwin Cosmetic preserving as much natural lakeshore Fertilizer By-law vegetation as possible. -

PP2 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB Trout Total L = Bait Allowed Arm Lake OPEN MAY 15 to MAR
Legend: As examples, ‘3 over 63 cm’ indicates a possession and size limit of ‘3 fish each over 63 cm’ or ‘10 fish’ indicates a possession limit of 10 for that species of any size. An empty cell indicates the species is not likely present at that waterbody; however, if caught the default regulations for the Watershed Unit apply. SHL=Special Harvest Licence, BKTR = Brook Trout, BNTR=Brown Trout, BURB = Burbot, CISC = Cisco, CTTR = Cutthroat Trout, DLVR = Dolly Varden, GOLD = Goldeye, LKTR = Lake Trout, LKWH = Lake Whitefish, MNWH = Mountain Whitefish, NRPK = Northern Pike, RNTR = Rainbow Trout, SAUG = Sauger, TGTR = Tiger Trout, WALL = Walleye, YLPR = Yellow Perch. Regulation changes are highlighted blue. Waterbodies closed to angling are highlighted grey. PP2 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB Trout Total l = Bait allowed Arm Lake OPEN MAY 15 TO MAR. 31 l 3 over 15 fish 63 cm Battle Lake Portion west of the west boundary of section 22-46-2-W5; OPEN JUNE 1 TO MAR. 31 l 0 fish 0 fish 5 fish 10 fish 2 fish; but limit Includes tributaries and outlet downstream to Sec. Rd. 771 is 0 from Feb. 1 to Mar. 31 Remainder of the lake OPEN MAY 15 TO MAR. 31 l 0 fish 0 fish 5 fish 10 fish 2 fish; but limit is 0 from Feb. 1 to Mar. 31 Berry Creek (Carolside) Reservoir 27-12-W4 OPEN MAY 15 TO MAR. 31 l 1 fish 45-50 1 fish 15 fish cm 63-70 cm Big Lake Includes tributaries OPEN MAY 15 TO MAR. -

LAND USE BYLAW WATERSHED REGULATIONS a Discussion Guide for Pigeon Lake Municipalities
LAND USE BYLAW WATERSHED REGULATIONS A Discussion Guide for Pigeon Lake Municipalities Prepared By: Municipal Planning Services Prepared For: Pigeon Lake Watershed Management Plan (PLWMP) Steering Committee Draft Date: August 2020 P a g e 1 This page is left intentionally blank TABLE OF CONTENTS TABLE OF CONTENTS I OPPORTUNITIES 11 ACKNOWLEDGEMENTS 1 IMPLEMENTATION 3: CLEAN RUNOFF PRACTICES 12 PLWMP STEERING COMMITTEE 1 EXISTING CONDITIONS 12 MUNICIPALITIES 1 MUNICIPAL AUTHORITY 12 PIGEON LAKE WATERSHED ASSOCIATION (PLWA) 1 CURRENT LAND USE BYLAW REGULATIONS 13 CONSULTING SERVICES 1 OPPORTUNITIES 14 ABOUT PIGEON LAKE 2 IMPLEMENTATION 4: GROUNDWATER QUALITY 15 GEOGRAPHY AND DEVELOPMENT HISTORY 2 EXISTING CONDITIONS 15 PIGEON LAKE WATERSHED MANAGEMENT PLAN 3 MUNICIPAL AUTHORITY 15 OTHER STUDIES AND REPORTS 3 CURRENT LAND USE BYLAW REGULATIONS 16 OPPORTUNITIES 17 PROJECT OVERVIEW 4 IMPLEMENTATION 5: SHORELINE AND RIPARIAN AREAS 18 PURPOSE & OBJECTIVES 4 Part 1: Discussion Guide - Complete 4 EXISTING CONDITIONS 18 Part 2: Engagement – To be rescheduled due to COVID-19 4 MUNICIPAL AUTHORITY 18 Part 3: Implementation Guides – MPS to prepare and present to Councils CURRENT LAND USE BYLAW REGULATIONS 19 following Engagement 4 OPPORTUNITIES 20 LIMITATIONS & APPLICABILITY 4 CURRENT REGULATORY ENVIRONMENT & SUCCESSES 4 SUMMARY 21 SIGNIFICANCE OF MUNICIPAL REGULATIONS AND BYLAWS 5 APPENDIX A: LIST OF LAND USE BYLAWS 22 METHODOLOGY 5 APPENDIX B: ACRONYMS & DEFINITIONS 23 IMPLEMENTATION 1: LAND COVER AND BIODIVERSITY 6 ABBREVIATIONS 23 EXISTING CONDITIONS -

2012 Pigeon Lake Regional Chamber of Commerce Members
2012 Pigeon Lake Regional Chamber of Commerce Members NAME MAIL ADDRESS TOWN/CITY PO CODEPHONE PHONE 2 FAX CONTACT 4H Foundation of Alberta RR # 1 Westerose T0C 2V0 (780) 682-2153 (780) 682-3784 Bruce Banks Ahlstrom Wright Oliver & Cooper 200, 80 Chippewa Road Sherwood Park T8A 4W6 (780) 464-7477 (780)389-3559 Lee Ahlstrom AJ's Trading Post RR # 1 - 47404 Hwy 778 Thorsby T0C 2P0 (780) 389-3797 (780) 389-4414 Antje Anderson Alberta Works 5201 - 51 Avenue Wetaskiwin T9A 0V5 (780) 360-9111 (780)361-1370 Moyra Kennedy Apollo Concrete 5737 50 St., P.O. Box 579 Warburg T0C 2T0 (780) 848-7766 (780)898-2586 (780) 848-7795 Evonne Hoople ATB Financial 5202 - 50 Avenue Wetaskiwin T9A 0S8 (780) 361-5191 (780) 352-0907 ATB Financial Westerose Agency 30 Village Drive, RR # 2 Westerose T0C 2V0 (780) 586-3803 (780) 586-3801 Sharon Will Backstreet Gifts & Antiques Ltd RR # 2, Box 105, Hwy 13 Westerose T0C 2V0 (780) 586-0733 (780) 586-0730 Elona Bonnett & Maureen Tabler Bayview Grocery & Liquor Locker 5602 - 49 Street, Box 77 Mulhurst Bay T0C 2C0 (780) 389-3784 (780) 389-4097 Jongshin, Suh (Joanne) Beachside Docks P.O. Box 117 Westerose T0C 2V0 (780) 361-5569 (780)586-2874 Wade Stephan & Landen Stephan Beachside Landscaping P.O. Box 117 Westerose T0C 2V0 (780) 361-5569 (780) 586-2874 Wade Stephan & Landen Stephan Black Bull Golf Resort P.O. Box 9 Ma-Me-O-Beach T0C 1X0 (780) 586-2254 (780) 586-2597 Judy Wylie Black Dog Drilling & Environmental Services Ltd.