Functional Aspect of Colostrum and Whey Proteins in Human Milk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
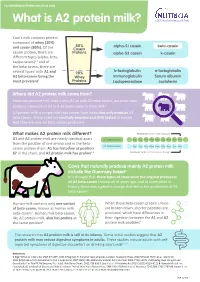
What Is A2 Protein Milk?
For Healthcare Professional use only What is A2 protein milk? Cow’s milk contains protein composed of whey (20%) 80% alpha-S1 casein beta-casein and casein (80%). Of the Casein casein protein, there are Proteins alpha-S2 casein k-casein different types (alpha, beta, kappa casein)1,2 and of the beta-casein, there are b-lactoglobulin a-lactoglobulin several types’ with A1 and 20% A2 beta-casein being the Whey Immunoglobulin Serum albumin most prevalent3 Proteins Lactoperoxidase Lactoferrin Where did A2 protein milk come from? Cows can produce milk that is only A1 or only A2 beta-casein, but most cows produce a mixture of A1 and A2 beta-casein in their milk4 A2 protein milk is simply milk that comes from cows that only produce A2 beta-casein. These cows are carefully selected and DNA tested to ensure that they are only A2 beta-casein producers What makes A2 protein milk different? Position 67(proline hinders cleavage) A1 and A2 protein milk are nearly identical apart A2 beta-casein Val Tyr Pro Phe Pro Gly Pro Iie Pro from the position of one amino acid in the beta- A1 beta-casein casein protein chain: A1 has histadine at position Val Tyr Pro Phe Pro Gly Pro Iie His 67 in the chain, and A2 protein milk has proline4,5 Position 67(histidine readily allows cleavage) Cows that naturally produce mainly A2 protein milk include the Guernsey breed4 It is thought that these types of cows were the original producers of A2 beta-casein thousands of years ago, and at some point in history there was a genetic change that led to the production of A1 beta-casein6 Human milk contains only one variant When these beta-casein protein chains of beta-casein, known as human milk are broken down, shorter peptides are beta-casein7. -

Casein Proteins As a Vehicle to Deliver Vitamin D3
Casein Proteins as a Vehicle to Deliver Vitamin D3: Fortification of Dairy Products with vitamin D3 and Bioavailability of Vitamin D3 from Fortified Mozzarella Cheese Baked with Pizza by Banaz Al-khalidi A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Nutritional Sciences University of Toronto © Copyright by Banaz Al-khalidi (2012) Casein proteins as a vehicle to deliver vitamin D3: Fortification of dairy products with vitamin D3 and Bioavailability of vitamin D3 from fortified Mozzarella cheese baked with pizza Banaz Al-khalidi Master of Science Department of Nutritional Sciences, Faculty of Medicine University of Toronto 2012 ABSTRACT Current vitamin D intakes in Canada are inadequate. The extension of vitamin D fortification to additional foods may be an effective and appropriate strategy for increasing vitamin D intakes in the general population. Cheese is potentially an ideal candidate for vitamin D fortification. We introduce the potential use of casein proteins as a vehicle for vitamin D3 fortification in industrially made cheeses where we found that over 90% of vitamin D3 added to milk was retained in both Cheddar and Mozzarella cheeses. Use of casein proteins for vitamin D3 fortification did not fully prevent vitamin D3 loss into whey. However the loss was minimized to approximately 8%. We then show that vitamin D3 is bioavailable from fortified Mozzarella cheese baked with pizza suggesting that the high temperature baking process does not significantly breakdown vitamin D3. Our findings could have important implications in increasing fortified food options for Canadians. ii TABLE OF CONTENTS ABSTRACT .................................................................................................................................. -

Breastfeeding Is Best Booklet
SOUTH DAKOTA DEPARTMENT OF HEALTH WIC PROGRAM Benefits of Breastfeeding Getting Started Breastfeeding Solutions Collecting and Storing Breast Milk Returning to Work or School Breastfeeding Resources Academy of Breastfeeding Medicine www.bfmed.org American Academy of Pediatrics www2.aap.org/breastfeeding Parenting website through the AAP www.healthychildren.org/English/Pages/default.aspx Breastfeeding programs in other states www.cdc.gov/obesity/downloads/CDC_BFWorkplaceSupport.pdf Business Case for Breastfeeding www.womenshealth.gov/breastfeeding/breastfeeding-home-work- and-public/breastfeeding-and-going-back-work/business-case Centers for Disease Control and Prevention www.cdc.gov/breastfeeding Drugs and Lactation Database (LactMed) www.toxnet.nlm.nih.gov/newtoxnet/lactmed.htm FDA Breastpump Information www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ HomeHealthandConsumer/ConsumerProducts/BreastPumps Healthy SD Breastfeeding-Friendly Business Initiative www.healthysd.gov/breastfeeding International Lactation Consultant Association www.ilca.org/home La Leche League www.lalecheleague.org MyPlate for Pregnancy and Breastfeeding www.choosemyplate.gov/moms-pregnancy-breastfeeding South Dakota WIC Program www.sdwic.org Page 1 Breastfeeding Resources WIC Works Resource System wicworks.fns.usda.gov/breastfeeding World Health Organization www.who.int/nutrition/topics/infantfeeding United States Breastfeeding Committee - www.usbreastfeeding.org U.S. Department of Health and Human Services/ Office of Women’s Health www.womenshealth.gov/breastfeeding -

Colostrum MFT® Colostrum Microfiltrate
PHARMANEX® PRODUCT INFORMATION PAGE Colostrum MFT® Colostrum Microfiltrate SUPPORTS HEALTHY IMMUNE SYSTEM Positioning Statement occasional sleeplessness, exercise training and fatigue, or poor diet can Colostrum MFT® is a proprietary extract of bovine colostrum, clinically diminish the body’s production of immune-fighting proteins. Production can also decrease with age by as much as 10% per decade after age 50. shown to boost primary immune functions by providing a concentrated spectrum of supplemental immune antibody proteins.* Our immune systems can be further challenged by international travel and commerce that increase our exposure to a global network of potentially Concept harmful microorganisms. Recent clinical studies show that regular consumption A baby’s immune system at birth is vulnerable to the unfriendly of bovine colostrum may strengthen our immune system by providing mircoorganisms of a new environment. Nature provides a natural defense supplemental quantities of immune proteins.* 1 against potentially harmful organisms and toxins in the first milk, called colostrum, provided by a baby’s mother. Colostrum is rich in Colostrum MFT® is derived from high quality, standardized extract of bovine immunoglobulins and other active immune components that provide first-line colostrum that took twelve years and $50 million to develop. Derived using defense against invading microorganisms and stim-ulate the immune system a patented micro-filtration process, Colostrum MFT® has an extract to action by passing on important immune information from mother to child.* standardized to 40% IgG content, making Colostrum MFT® many times more concentrated than other colostrum products on the market today. Each The immune compounds of colostrum include special proteins and messenger daily dose of Colostrum MFT® delivers 500 times the IgG concentration of molecules that work with the body's own immune sys-tem to promote optimal raw milk and provides critical supplemental immune support to your body's own health and wellness. -

Collection and Storage of Colostrum
James Paget University Hospitals NHS Foundation Trust Collection and storage of colostrum Information for Patients Expressing and storage of your colostrum Breastmilk is beneficial to a newborn because it contains the correct nutrients and protective factors for the baby’s age. Colostrum is the milk produced in the first few days after birth. It is a concentrated fluid and comes in small amounts so your baby’s immature digestive system and kidneys can absorb the nutrients. Colostrum also has a laxative effect on the newborn which helps to minimise jaundice by assisting in the passing of meconium (your baby’s first stools). Most babies need some time to learn how to attach and feed well from the breast. The purpose of expressing and storing colostrum prior to birth is for those babies who may be at risk of low sugar levels or mothers who may need to stimulate their milk supply. This information has been provided for women who may have the following conditions and situations: • Women with diabetes in pregnancy (pre-existing or gestational) • Babies diagnosed with cleft lip or palate during the antenatal period • Women with breast hypoplasia (a condition where there is insufficient glandular breast tissue, making breastfeeding difficult) • Women with polycystic ovarian disease • Women who have multiple sclerosis • Women who have had breast surgery affecting milk ducts • A strong family history of dairy intolerance or inflammatory bowel disease. It is our intention to help you to plan for your baby’s birth and support you through the process of expressing your colostrum. This information will help you decide whether you wish to obtain your colostrum, but if you have any questions, you can contact: Our Infant Feeding Coordinator: 01493 453076 2 Breast feeding and Diabetes Research shows that babies who are breastfed are less likely to develop childhood diabetes. -

Antenatal Colostrum Collection Leaflet
Antenatal colostrum collection Information for pregnant women from the Infant Feeding Team This leaflet aims to provide a useful introduction for pregnant women on how to collect and store colostrum until baby arrives. If after reading this leaflet you still have questions, please speak to your midwife who will be able to answer your questions. What is colostrum? Why is it good for my baby? The first breast milk your body makes is known as colostrum and it is produced from about 16 weeks of pregnancy. It is usually a golden yellow colour and often very sticky. It is very easy for baby to digest and therefore the perfect first food for baby. It is concentrated in nutritional properties, so baby will only need small amounts in the early days. • Colostrum is full of antibodies to help protect your baby from infections. • It has a laxative effect, to help baby pass the early stools. • It can reduce the effects of jaundice in your baby. • Giving baby colostrum is a good way to help to regulate their blood sugar levels. 1 of 5 Antenatal colostrum collection (June 2021) Why are you recommending that I should collect my colostrum before my baby is born? Collecting colostrum before your baby is born can be helpful if we think your baby may have challenges with breastfeeding or keeping their blood sugar levels in their early days. This might be because: • your baby is large or small for their gestational age • you are expecting twins or triplets • your baby has a cleft lip or palate; or • they have a heart or other diagnosed condition. -

Gluten Free Casein Free Diet
GLUTEN FREE, CASEIN FREE DIET The gluten free, casein free (GFCF) diet has been shown to be helpful for individuals with allergies to these particular foods and specifically in the management of autistic spectrum disorder (ASD). Proteins found in grain and dairy products, known as gluten and casein respectively, are believed to be poorly broken down in the digestive tracts in some people. When these proteins are not digested properly they can be absorbed intact into blood circulation. These proteins can affect the brain by crossing the blood-brain barrier and binding to opioid receptors. This can affect mood, concentration, mental performance and pain tolerance (i.e. in autistic children this will increase their pain threshold). Research has shown significant improvement in several conditions, including schizophrenia and autism, following a GFCF diet. In a survey of over 3500 parents of autistic children, it was reported that 70% found a GFCF diet improved behavior, eye contact and socialisation, concentration and learning. It is recommended to follow the GFCF diet strictly for at least 6 months to assess the benefit of this diet. Below is a list of foods containing gluten and casein that are suggested to avoid, plus a list of alternative GFCF choices. RECOMMENDED AVOID GRAINS AND • Amaranth • Baked Beans unless gluten free LEGUMES • Basmati Rice • Flours: Wheat flour, wholemeal flour, • Beans bakers flour, semolina, barley, rye • Brown Rice (avoid battered or crumbed food) • Buckwheat • Wheat including durum, semolina, • Chickpea triticale, -

A2 Milk Popularity on the Rise by BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST
A2 Milk Popularity on the Rise BY BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST A hot topic in the dairy industry today is the Beta-casein protein production is controlled by the growing popularity of A2 beta-casein milk among combination of any two of these variants (ie. A1A2) as consumers and dairy farmers. Farmers in many all cows carry two alleles. These alleles are co-dominant, regions of the world are being incentivised to meaning that cows that carry two different variants produce A2 milk to meet the growing demand in (heterozygous) will produce equal amounts of each what is considered to be a healthier alternative protein that they carry, while cows that carry two copies to conventional dairy (Zoetis, 2015). However the of the same allele (homozygous) will produce only science behind this trend remains controversial that protein (Woodford, 2007). This makes achieving and is not well understood by many consumers a homozygous A2 herd exclusively through genetic and producers. The goal of this article is to selection a possibility for dairy producers. While a present an assessment of the facts as they are quick conversion to A2 would be possible via genetic currently known and explain Semex’s A2 brand. testing and selective culling of A1 carriers, a more sound approach could be a step-wise approach of genetic Milk is composed of several solid components including selection for A2A2 sires in advance of conversion to minerals, lactose, fat and protein. There are three notable mitigate the need for A1 culling. casein milk proteins: alpha, kappa, and beta-casein - the protein of interest to us in this article (Zoetis, 2015). -

Hidden Dairy “Cheat Sheet” Business Card-Sized (Cut out and Fold in the Middle)
From kellymom.com… Hidden Dairy “Cheat Sheet” Business card-sized (cut out and fold in the middle) Personal use only. If you would like to distribute this handout to clients or patients, please visit www.kellymom.com/bookstore/handouts for more information. Dairy Ingredients and Hidden Dairy Ingredients that MAY contain milk protein: Artificial butter flavor, Butter, Butter fat, Buttermilk, Chocolate, Flavorings (natural or artificial), High protein Butter oil, Casein, Caseinates (ammonia, calcium, flour, Hot Dogs, Luncheon Meat, Margarine, Simplesse, magnesium, potassium, sodium), Cheese, Cottage Sausage, Starter Distillate. cheese, Cream, Curds, Custard, Ghee, Goat’s milk, Avoid "deli" meats, because the slicers frequently are used Half & half, Hydrolysates (casein, milk protein, protein, to cut both meat and cheese products. Also, some deli whey, whey protein), Kefir, Koumiss, Lactalbumin, meats contain dairy products. Lactalbumin phosphate, Lactoglobulin, Lactose, Lactulose, Milk (condensed, derivative, powder, dry, Kosher labeling: A product label marked Parve or evaporated, low fat, malted, non fat, protein, skim, solids, Pareve is certified dairy-free. A product with a circled whole), Milkfat, Nougat, Paneer, Pudding, Rennet “U” on the label (with NO other symbols or letters) is casein, Sour cream, Sour cream solids, Sour milk solids, Parve. A "D" or "DE" on a product label next to a circled Whey (in any form including delactosed, demineralized, "K" or circled "U" may indicate the presence of milk protein concentrate, sweet), Yogurt protein. — kellymom.com . -

Hand Expressing in Pregnancy and Colostrum Harvesting—Preparation for Successful Breastfeeding?
clinical practice Hand expressing in pregnancy and colostrum harvesting—preparation for successful breastfeeding? Colostrum harvesting—what is it Abstract and why do it? Colostrum harvesting is a process involving antenatal expressing and Colostrum is a clear or yellow thick fluid, which storing of colostrum. Its benefits include quicker establishment of is rich in proteins, self-digesting fats, hormones, ‘full lactation’ (Singh, 2009), increased confidence in hand expressing enzymes, vitamins, minerals and immunoglobins, (Brisbane and Giglia, 2013), and reduced stress over breast milk supply and is excreted by the breasts in pregnancy, during in the immediate postpartum period (Cox, 2006). Despite its advantages, the process of Lactogenesis I. Human milk, unlike the use of colostrum harvesting remains limited, with only a small infant formula, which is standardised within a selection of UK Trusts currently utilising this process. In the past, there very narrow range of composition, is dynamic have been concerns over the safety of antenatal expressing and its and uniquely suited to each baby. Its nutritional potential to initiate premature labour (Soltani and Scott, 2012). A more composition and non-nutritive bioactive factors recent critical review of the literature found that the substantial benefits promote survival and healthy development of early feeding of colostrum outweigh the lack of evidence associated (Ofteda, 2012; Ballard and Morrow, 2013). with the risk of preterm labour (Chapman et al, 2013; East et al, 2014). Colostrum is produced in small quantities, This article discusses the advantages of antenatal hand expressing and but it is rich in immunologic components, colostrum harvesting in view of the best available evidence. -

The Effect of Bovine Colostrum on the Lactic Flora of Yogurt and Kefir
Central JSM Biotechnology & Biomedical Engineering Bringing Excellence in Open Access Research Article *Corresponding author Ahmet AYAR, Food Engineering Department, Sakarya The Effect of Bovine Colostrum University, Sakarya, Turkey, Tel: 905449167554; E-mail: Submitted: 21 April 2016 on the Lactic Flora of Yogurt Accepted: 05 October 2016 Published: 06 October 2016 ISSN: 2333-7117 and Kefir Copyright Ahmet Ayar*, Hatice Sıçramaz, and İmren Çetin © 2016 Ayar et al. Department of Food Engineering, Sakarya University, Turkey OPEN ACCESS Keywords Abstract • Colostrum The objective of this study was to determine the effect of colostrum on microbial • Yogurt populations of yogurt and kefir. For this purpose, raw bovine colostrum is freeze-dried • Kefir and added to yogurt and kefir on 8% and 16% (w/w; colostrum/product) dilutions. • Lactic acid bacteria The results showed that, effect of colostrum on total mesophilic aerobic bacteria counts of yogurt and kefir are negligible. Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus counts were 0.26-0.29 log CFU/g and 0.38-0.67 log CFU/g higher in colostrum added yogurt samples, respectively. In kefir, lactic streptococci and lactobacilli counts were higher than that of yogurt. However, they weren’t affected from colostrum addition, statistically. This study showed that, colostrum, which contains antimicrobial substances (immunoglobulins, lactoferrin, lactoperoxidase, lysozyme and cytokines), don’t have an adverse effect on specific microbial floras of fermented dairy products such as yogurt and kefir. As a result, colostrum can be added to yogurt and kefir to increase their functional properties. ABBREVIATIONS LAB: Lactic Acid Bacteria; CFU: Colony-Forming Unit microorganisms. Lysozyme is a potent antimicrobial enzyme but, INTRODUCTION in contrast to human milk, the concentration in bovine milk and colostrum is probably too low to significantly contribute to the overall bacteriostatic and bactericidal activity [3]. -

Evaluation of Immunoglobulin G Absorption from Goat Colostrum by Newborn Piglets
animals Article Evaluation of Immunoglobulin G Absorption from Goat Colostrum by Newborn Piglets Silvia Martínez Miró * , Susana Naranjo , Josefa Madrid, Miguel José López, Cristian Jesús Sánchez, Mónica Marcela Segura and Fuensanta Hernández Department of Animal Production, Faculty of Veterinary, University of Murcia, Campus de Espinardo, 30100 Murcia, Spain; [email protected] (S.N.); [email protected] (J.M.); [email protected] (M.J.L.); [email protected] (C.J.S.); [email protected] (M.M.S.); [email protected] (F.H.) * Correspondence: [email protected]; Tel.: +34-868-884-934 Received: 11 March 2020; Accepted: 5 April 2020; Published: 7 April 2020 Simple Summary: Alternatives to sow colostrum are necessary to ensure adequate colostrum intake by piglets born from hyperprolific sows. This study was conducted to evaluate whether piglets can absorb goat immunoglobin G (IgG) and study its effects on piglets. The results showed that piglets absorbed goat IgG with an apparent coefficient of absorption of 20.9%. In addition, the piglets tolerated goat colostrum well, opening up the possibility of developing supplements based on goat colostrum for newborn piglets. Abstract: The aim of this study was to evaluate whether piglets absorb immunoglobin G (IgG) from goat colostrum and the potential effects of its ingestion on suckling piglets. Thirty-eight piglets with body weights ranging from 1000 to 1700 g were assigned to one of the three experimental treatments: Control group (C), where piglets were allowed to suckle normally, and porcine and goat groups. The piglets from the last two groups were removed from the sows after birth and received an oral 20 mL dose every 3 h of porcine (PC) or goat colostrum (GC), respectively, during first 12 h of life.