Ciprofloxacin-Loaded Bioadhesive Hydrogels for Ocular Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Chloride Injection USP 0.9% Prefilled Syringes, for Intravascular Use Only ------WARNINGS and PRECAUTIONS ------Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION --------------------- DOSAGE FORMS AND STRENGTHS --------------------- These highlights do not include all the information needed to use • Supplied as a clear, colorless, odorless, sterile solution of Sodium Sodium Chloride Injection USP 0.9% safely and effectively. See Chloride 0.9% for intravenous administration (3) full prescribing information for Sodium Chloride Injection USP • Supplied in 50 mL and 125 mL prefilled syringes (3) 0.9%. ------------------------------- CONTRAINDICATIONS ------------------------------- • None (4) Sodium Chloride Injection USP 0.9% Prefilled Syringes, For Intravascular Use Only ----------------------- WARNINGS AND PRECAUTIONS ----------------------- Initial U.S. Approval: 2006 • Remove all air from the syringe and associated tubing prior to injection. (5.1) ----------------------------INDICATIONS AND USAGE ---------------------------- • For use in flushing compatible contrast agents through Liebel- • May cause fluid overload in patients with congestive heart failure, Flarsheim intravenous administration sets into indwelling severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia. (5.3) intravascular access devices (1) • Establish intravascular catheter patency prior to administration. ----------------------- DOSAGE AND ADMINISTRATION ----------------------- (5.4) • For single patient use only. (2) • Determine the volume of flush based on the imaging procedure, ------------------------------ ADVERSE REACTIONS ------------------------------ -

Aerogen Nebulizer)
Page 1 of Date of Original: 11/15/18 Date of Review: Date of Revision: 9/4/19 Respiratory Therapy POLICY & PROCEDURE MANUAL RIBAVIRIN ADMINISTRATION (Aerogen nebulizer) PURPOSE: 1. Standardize the administration and delivery of ribavirin by aerosol 2. To protect the patient and caregivers by standardizing precautions for ribavirin delivery POLICY: The patient room must be set up according to St Jude’s Environmental Health and Safety policy prior to the administration of Ribavirin. Room preparation is to be done by the nursing staff. *See procedure section below https://home.stjude.org/ehs/Pages/ribavirin-precautions.aspx • Negative pressure room is mandatory for Ribavirin administration. • Preparation of the room is the responsibility of the nursing staff. • Ribavirin will be delivered from pharmacy in primed Aerogen syringe set appropriate for use on the MedFusion 3500 syringe pump. • The syringe pumps will be used, maintained, and stored by the respiratory therapy department. • The PPE cart is the shared responsibility of the respiratory therapist who administers the first dose of ribavirin and the nursing coordinator for that area. • Proper sign including contact precautions and STOP will be shared responsibility of the nursing and respiratory care. • All instances of spilled or leaked ribavirin must be documented in the event reporting system. • Ribavirin may be delivered to a patient during mechanical ventilation in a very specific set of circumstances. The final decision on the use of ribavirin is at the discretion of the ICU physician on service. EQUIPMENT: • Negative pressure room • DeMistifier 2000 HEPA filtration system. • DeMistifier negative pressure bed enclosure with canopy. • MedFusion 3500 Syringe pump • Flowmeter • Aerogen vibrating mesh nebulizer • Delivery Supplies (O2 tubing, O2 reducer, large bore tubing, T adaptor, and aerosol face mask) • Prescribed medication • STOP sign for the door during drug administration PPE • N95 or Positive Air Pressure mask required • Chemo resistant Gown • Double Gloves • Shoe covers PROCEDURE: 1. -

IFU for Bioglue Surgical Adhesive (Outside USA)
Instructions for Use Syringe BioGlue® Adhésif BioGlue® Collante Chirurgico Adhesivo Quirúrgico Chirurgical Mode Chirurgischer Klebstoff BioGlue® Istruzioni d’uso BioGlue® Instrucciones d’emploi Seringue Gebrauchsanwei sung Siringa de Uso Jeringuilla Spritze BioGlue® Adesivo BioGlue® Kirurgisk BioGlue® Kirurgisk Lim BioGlue® Vävnadslim Cirúrgico Instruções de Klæbemiddel Bruksanvisning Sprøyte Bruksanvisning Spruta Utilização Seringa Brugsanvisning Sprøjte Χειρουργική συγκολλητική BioGlue® Cerrahi Chirurgické adhezivum BioGlue® ουσία BioGlue® Οδηγίες Yapışkan Kullanım BioGlue® Návod k použití Chirurgische Kleefstof χρήσης Σύριγγα Talimatları Şırınga Stříkačka Gebruiksinstructies Spuit Chirurgické Lepidlo BioGlue® Sebészi Klej Chirurgiczny Хирургический клей BioGlue® Návod na Szövetragasztó BioGlue® Sposób Użycia BioGlue® Инструкция по Použitie Striekačka Használati útmutató Strzykawka применению Шприц Fecskendő CryoLife, Inc. 1655 Roberts Boulevard, NW • Kennesaw, Georgia 30144 • USA • +1 (770) 419-3355 • Fax +1 (770) 590-3753 http://www.cryolife.com 25°C 0°C L6313.009 Rev (07/2010) ENGLISH PRODUCT DESCRIPTION BioGlue® Surgical Adhesive is composed of bovine serum albumin and glutaraldehyde. The product’s two components are mixed within the applicator tip of the syringe during application to tissue. Polymerization of the surgical adhesive begins immediately upon application, reaching bonding strength within 2 minutes. INDICATIONS • Do not peel away BioGlue from an unintended site, as 3. While firmly grasping the syringe, nose upward, BioGlue Surgical Adhesive is indicated for use as an this could result in tissue damage. turn the cap 90° counterclockwise and remove the adjunct to standard methods of surgical repair (such • An animal study12 has shown that the circumferential cap by rocking it from side-to-side. Align the tip with as sutures, staples, electrocautery, and/or patches) to application of BioGlue restricts dilatation on growing the syringe using the corresponding notches on bond, seal, and/or reinforce soft tissue. -

Veterinary Anesthetic and Analgesic Formulary 3Rd Edition, Version G
Veterinary Anesthetic and Analgesic Formulary 3rd Edition, Version G I. Introduction and Use of the UC‐Denver Veterinary Formulary II. Anesthetic and Analgesic Considerations III. Species Specific Veterinary Formulary 1. Mouse 2. Rat 3. Neonatal Rodent 4. Guinea Pig 5. Chinchilla 6. Gerbil 7. Rabbit 8. Dog 9. Pig 10. Sheep 11. Non‐Pharmaceutical Grade Anesthetics IV. References I. Introduction and Use of the UC‐Denver Formulary Basic Definitions: Anesthesia: central nervous system depression that provides amnesia, unconsciousness and immobility in response to a painful stimulation. Drugs that produce anesthesia may or may not provide analgesia (1, 2). Analgesia: The absence of pain in response to stimulation that would normally be painful. An analgesic drug can provide analgesia by acting at the level of the central nervous system or at the site of inflammation to diminish or block pain signals (1, 2). Sedation: A state of mental calmness, decreased response to environmental stimuli, and muscle relaxation. This state is characterized by suppression of spontaneous movement with maintenance of spinal reflexes (1). Animal anesthesia and analgesia are crucial components of an animal use protocol. This document is provided to aid in the design of an anesthetic and analgesic plan to prevent animal pain whenever possible. However, this document should not be perceived to replace consultation with the university’s veterinary staff. As required by law, the veterinary staff should be consulted to assist in the planning of procedures where anesthetics and analgesics will be used to avoid or minimize discomfort, distress and pain in animals (3, 4). Prior to administration, all use of anesthetics and analgesic are to be approved by the Institutional Animal Care and Use Committee (IACUC). -

Nurseadviseerr201805.Pdf
May 2018 Volume 16 Issue 5 Safety with nebulized medications requires an interdisciplinary team approach Consider implementing the following recom - ebulization of inhaled medications provides an excellent delivery route for mendations to reduce the risk of errors with the treatment of both pulmonary and nonpulmonary conditions. The mist is nebulized medications. Neasily inhaled by breathing normally, without requiring coordinated breaths to inhale the medication (as required with metered dose inhalers). This delivery Communicate orders. Establish a reli - method generally provides a rapid onset of drug action, and minimizes systemic ef - able system for communicating new and fects. An additional advantage is that the nebulizer is adaptable and can be used changed orders for nebulized medications with a mouthpiece, adult or pediatric face mask, tracheostomy collar, T piece, or to respiratory therapy staff. Test the system ventilator circuit. before use, monitor its reliability, and inves - tigate all failed communication of orders to Unfortunately, there can also be errors with nebulized medications that are linked to determine opportunities for improvement. a wide variety of common system failures and human factors. However, review of errors reported to the ISMP National Medication Errors Reporting Program (ISMP Collaborate on assessments. Consider MERP) and other sources during 2016 and 2017, revealed patterns among error types collaboration between nursing and respi - and causative factors that were relatively unique to nebulized medications and/or ratory staff to determine if a dose of neb - the conditions under which they are prescribed, dispensed, and administered. ulized medication should be held based on an assessment of the patient. Deter - Omissions due to unavailability of respiratory therapist or failed communication mine conditions that require prescriber By far, the most frequently reported errors related to the use of nebulized medications notification of a held nebulizer treatment. -

Medfusion® 3500 Syringe Infusion Pump with Pharmguard® Medication Safety Software
Medfusion® 3500 Syringe Infusion Pump with PharmGuard® Medication Safety Software Smart and simple with added safety The Department of Health estimates we will be facing a shortage of over 800,000 nurses by the year 2020.1 Current shortages are having serious effects on day-to-day healthcare, and many nurses feel overworked and stressed – a situation that may lead to medication errors. The Joint Commission estimates 25% of hospitalized patient deaths are related to nurse understaffing.2 The dangers of medication errors n As many as 19 patients die every day from ® MR Conditional: The Medfusion pump may be used in static 3 magnetic fields up to 150 Gauss when secured to a non-movable medication errors in the United States object. Tested using 1.5 Tesla 64MHz shielded MRI system. n More than 770,000 injuries and deaths are due to preventable drug errors, which cost 3 hospitals up to $5.6 million per year n 61% of serious and life-threatening errors 3 are associated with infusion therapy n Infusion devices account for up to 35% of all medication errors, with 67% due to 4 programming errors n 38% of medication errors occur during the administration process; 51% harmed 5 the patient 1 National Center for Workforce Analysis, U.S. Department of Health and Human Services 2 The Joint Commission, 2002 3 Materials Management in Health Care, Oct. 2005 4 Tourville, 2003 5 JAMA, 1995 The total solution Post-occlusion bolus reduction ® The Medfusion syringe pump removes the possibility of a safety system has been the leader patient receiving an automatic bolus following an occlusion in infusion pump technology for over 15 years. -

From Syringe to Autoinjector
Volume 40 Number 6 Blow-Fill-Seal Data Integrity Using Modular JUNE 2016 Volume 40 Number 6 PLUS: Technology Training Construction PHARMACEUTICAL TECHNOLOGY From Syringe to JUNE 2016 2016 JUNE Autoinjector Building a Better Self-Injection Solution PharmTech.com PEER-REVIEWED Qualifying Personnel to Visually Inspect Cleaned Equipment, Part II SINGLE -USE SYSTEMS FORMULATION API SYNTHESIS & MANUFACTURING Integrating Single-Use Systems Customizing HPMC Adoption of Continuous Processing Advancing Drug Delivery Through Superior Polymer Design Safe and effective delivery of today’s complex drug molecules is increasingly a function of collaboration between polymer experts and pharmaceutical product development teams. Acting on enabling science, producing superior polymer excipient technology, and creating systems for optimum bioavailability, Ashland works alongside formulators to advance drug- specific delivery platforms. Explore how you can solve intricate drug formulation and development challenges with polymer expertise from Ashland. Visit us at Ashland.com/pharmaceutical Come visit us at Booth 610 Ashland [email protected] www.ashland.com/pharmaceutical ®Registered trademark, Ashland or its subsidiaries, registered in various countries ™Trademark, Ashland or its subsidiaries, registered in various countries ©2016, Ashland AD-13475.1 “Off-the-shelf solutions? No thank you. After all, our customers are something special.” Daniel Drossel Mechanical Engineering Technician (Design department) Each customer has its very own special requirements. That’s why we, at Optima, manufacture fi lling lines that are fi ne-tuned to our clients’ particular needs while off ering the benefi ts of an integrated and complete line: The complete machine package including high-precision functionalities, backed by consistent documentation and supported by an optimized and tailored software solution – in addition to a central point of contact who is passionate about your every concern… We are experts in special solutions, after all. -

Effects of Different Polymers on Thermal Inkjet Printing of Oral Thin Films: a Novel Approach
Effects of Different Polymers on Thermal Inkjet Printing of Oral Thin Films: A Novel Approach A project submitted by Rubaiat Ahmed ID: 13146043 Session: Spring 2013 to The Department of Pharmacy in partial fulfillment of the requirements for the degree of Bachelor of Pharmacy (Hons.) Dhaka, Bangladesh September, 2018 This work is dedicated to my parents for their love and constant support. Certification Statement This is to certify that the project titled “Effects of Different Polymers on Thermal Inkjet Printing of Oral Thin Film: A Novel Approach” submitted for the partial fulfillment of the requirements for the degree of Bachelor of Pharmacy (Hons.) from the Department of Pharmacy, BRAC University constitutes my own work under the supervision of Marzia Alam, Lecturer, Department of Pharmacy, BRAC University and that appropriate credit is given where I have used the language, ideas or writings of another. Signed, __________________________________ Counter signed by the supervisor, __________________________________ Acknowledgement All praise is for the Almighty who is the source of our life and strength of our knowledge and wisdom, has helped me to continue my study in full diligence which I hope will reflect in my project. This research could not also have been completed without the support of many people who are gratefully acknowledged here. I am thankful to my honorable supervisor, Marzia Alam, Lecturer, Department of Pharmacy, BRAC University, for her amiability to provide me with untiring guidance, whole cooperation and for her extensive knowledge in research that helped me in all the spheres to perform the project work. I would like to put forward my most sincere regards and profound gratitude to Dr. -

How to Help…Your Child Take Medicine
How to Help…Your Child Take Medicine Having a child take medicine by mouth is not always an easy task; in fact having a child take medicine by mouth and having the situation remain positive and encouraging can seem, at times, impossible. Below are some ideas to help encourage children to take their oral medication while providing them with a sense of control. o Always prepare your child in advance when they need to take medicine by mouth. o Children are sensitive to the environment and emotions of others. They will quickly sense if you are expecting a lack of cooperation. Remain positive and encouraging. o Use play to help your child practice by encouraging them to give “medicine” to a doll or stuffed animal with an empty syringe or medicine cup. o When possible, give choices. (i.e. what would they like it mixed with, what would they like to drink before/after, how would they like to take it…in a cup, in a syringe). o Encourage the child to hold onto a security item. o To partially numb the taste buds, have the child suck on a Popsicle right before giving the medication. o Mixing medicine with a flavoring such as Kool-aid powder, chocolate syrup or maple syrup may mask a strong taste. o Chalky liquids often taste better by mixing them with chocolate or vanilla ice cream (always in amounts the child is guaranteed to finish). o Have your child “chase” the medicine with a favorite beverage such as juice, soda or chocolate milk. White grape juice is great for masking a bitter taste. -

Injection Guide Overview and Goals of This Guide ����������������������������������� 1
Injection Guide Overview and goals of this guide . 1 Overview of injections. 3 Why did my doctor choose an injectable medication? ��������������� 3 Differences between injectable medication and oral medication . 3 Types of injections. 4 Parts of a syringe ��������������������������������������������������� 4 Steps for injecting medication ��������������������������������������� 5 Storage ������������������������������������������������������������� 5 Preparation ��������������������������������������������������������� 6 Wash your hands ����������������������������������������������� 6 Prepare your medication for injection ��������������������������� 7 Prepare the injection site ��������������������������������������� 8 Injection ����������������������������������������������������������� 9 Intramuscular injection ����������������������������������������� 9 Subcutaneous injection ����������������������������������������10 Disposal. .11 Monitoring of the injection site ����������������������������� 12 References . .13 Injection-site records ������������������������������������������������14 Overview and goals of this guide This guide is a quick reference on how to use medications that are given by injection. It gives step-by-step instructions for preparing and injecting the medication. It also covers safety precautions. The instructions in this guide apply whether you are giving the injection to someone you care for or you are giving the injection to yourself. This guide is not meant to replace instructions from your doctor nor the information -
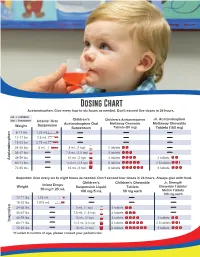
Dosing Chart Acetaminophen: Give Every Four to Six Hours As Needed
Dosing Chart Acetaminophen: Give every four to six hours as needed. Don’t exceed five doses in 24 hours. mL = milliliter Jr. Acetaminophen tsp = teaspoon Infants’ Oral Children’s Children’s Acetaminophen Acetaminophen Oral Meltaway Chewable Meltaway Chewable Weight Suspension Suspension Tablets (80 mg) Tablets (160 mg) 6-11 lbs. 1.25 mL 1.25 mL 1.25 mL 12-17 lbs. 2.5 mL 2.5 mL 1.25 mL 3.75 mL 18-23 lbs. 3.75 mL 2.5 mL 1.25 mL 3.75 mL 2.5 mL 5 mL 24-35 lbs. 5 mL 5 mL (1 tsp) 5 mL 2 tablets 36-47 lbs. 7.5 mL (1.5 tsp) 7.5 mL 3 tablets 48-59 lbs. 10 mL (2 tsp) 10 mL 4 tablets 2 tablets Acetaminophen 60-71 lbs. 12.5 mL (2.5 tsp) 12.5 mL 5 tablets 2.5 tablets 15 mL 72-95 lbs. 15 mL (3 tsp) 6 tablets 3 tablets Ibuprofen: Give every six to eight hours as needed. Don’t exceed four doses in 24 hours. Always give with food. Children’s Children’s Chewable Jr. Strength Infant Drops Weight Suspension Liquid Tablets Chewable Tablets/ 50 mg/1.25 mL 100 mg/5 mL 50 mg each Motrin Tablets 100 mg each 12-17 lbs. 1.25 mL 1.25 mL 1.25 mL 18-23 lbs. 1.875 mL 1.875 mL 24-35 lbs. 5 mL (1 tsp) 2 tablets 5 mL 36-47 lbs. 7.5 mL (1.5 tsp) 7.5 mL 3 tablets 48-59 lbs. -

IV Medicine at Home: Excelsior Syringe Pump (ESP)
IV Medicine at Home: Excelsior Syringe Pump (ESP) Patient Name : __________________________________ MRN ____________ DOB ____________ Medication Information Name of Medication: ______________ Dose: _____________ Medication stable at room temperature for:______________ Give medication every _________ hours Times to give medication: Pump speed Normal Slow Extra Slow Flush line with: Normal saline flush solution Dextrose flush solution Amount to give: __________ ml When to give it: before and after giving the medication ____________________ Heparin flush solution Solution: __________ units/ml Amount to give: __________ ml When to give it: After giving the medication and normal saline flush ________________________ © Children’s Minnesota • Patient & Family Education Revised 12/17 What is an Excelsior Syringe Pump (ESP)? 2. Check the label on the syringe and verify: The ESP is a small battery-powered medicine Child’s name delivery pump. A medicine-filled syringe, with a long, thin tube is attached to the ESP. The thin Medication/Dose tube is then attached to your child's IV and the Expiration date medicine is slowly pushed into the IV. Preventing infection Priming the tubing It is very important to prevent infection. Failure 1. Check for air bubbles in the medication to do so might require removal of the central syringe and remove them if needed. venous catheter. The nurse will show you how To remove air bubbles: remove the tip to keep your supplies clean, so no germs will cover from the syringe and point the enter the catheter and cause an infection. syringe upward, pull back slightly on plunger and squirt air bubble out. Cleanliness is a must! 2.