Study of Plants Used Against Infections by California Native
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
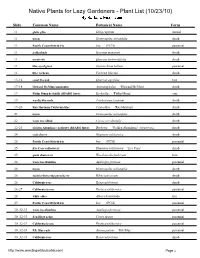
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Picrotoxin-Like Channel Blockers of GABAA Receptors
COMMENTARY Picrotoxin-like channel blockers of GABAA receptors Richard W. Olsen* Department of Molecular and Medical Pharmacology, Geffen School of Medicine, University of California, Los Angeles, CA 90095-1735 icrotoxin (PTX) is the prototypic vous system. Instead of an acetylcholine antagonist of GABAA receptors (ACh) target, the cage convulsants are (GABARs), the primary media- noncompetitive GABAR antagonists act- tors of inhibitory neurotransmis- ing at the PTX site: they inhibit GABAR Psion (rapid and tonic) in the nervous currents and synapses in mammalian neu- system. Picrotoxinin (Fig. 1A), the active rons and inhibit [3H]dihydropicrotoxinin ingredient in this plant convulsant, struc- binding to GABAR sites in brain mem- turally does not resemble GABA, a sim- branes (7, 9). A potent example, t-butyl ple, small amino acid, but it is a polycylic bicyclophosphorothionate, is a major re- compound with no nitrogen atom. The search tool used to assay GABARs by compound somehow prevents ion flow radio-ligand binding (10). through the chloride channel activated by This drug target appears to be the site GABA in the GABAR, a member of the of action of the experimental convulsant cys-loop, ligand-gated ion channel super- pentylenetetrazol (1, 4) and numerous family. Unlike the competitive GABAR polychlorinated hydrocarbon insecticides, antagonist bicuculline, PTX is clearly a including dieldrin, lindane, and fipronil, noncompetitive antagonist (NCA), acting compounds that have been applied in not at the GABA recognition site but per- huge amounts to the environment with haps within the ion channel. Thus PTX major agricultural economic impact (2). ͞ appears to be an excellent example of al- Some of the other potent toxicants insec- losteric modulation, which is extremely ticides were also radiolabeled and used to important in protein function in general characterize receptor action, allowing and especially for GABAR (1). -

NTP Annual Report 2012
National Toxicology Program U.S. Department of Health and Human Services Annual Report 2012 National Toxicology Program ANNUAL REPORT for Fiscal Year 2012 National Institute of Environmental Health Sciences National Institutes of Health National Center for Toxicological Research Food and Drug Administration National Institute for Occupational Safety and Health Centers for Disease Control and Prevention September 2013 Department of Health and Human Services National Toxicology Program NIH Publication No. 13-5970 Table of Contents Letter from the NIEHS/NTP Director ...................................................................................................... 1 1. National Toxicology Program: Mission And Goals ....................................................................... 3 A. Organizational Structure and Oversight ...................................................................................... 4 B. Training Programs ....................................................................................................................... 6 C. Advisory Boards and Committees ............................................................................................... 6 i. NTP Executive Committee .................................................................................................6 ii. NTP Board of Scientific Counselors ...................................................................................7 iii. Scientific Advisory Committee on Alternative Toxicological Methods .................................9 -

Erowid Extracts — Number 13 / November 2007 Erowid Extracts Table of Contents Number 13, November 2007
Erowid® Extracts D OCUMENTING THE C OMPLEX R ELATIONSHIP B ETWEEN H UMANS AN D P SYCHOACTIVES November 2007 Number 13 “The problem to be faced is: how to combine loyalty to one’s own tradition with reverence for different traditions.” — Abraham J. Heschel The Absinthe Enigma • Wormwood and Thujone • P. viridis vs. M. tenuiflora Varieties of Nicotine Experience • Khat Legal Challenges LETTERS & FEEDBACK Hi there Erowid staff, First of all, thank you for such Awesome website! A more thoughtfully a wonderful site. I’m not a serious compiled compendium of information I’m just writing to say how much I recreational user, but having some on the topic of psychoactives does appreciate your website. My father chronic pain issues, I tend to experiment not exist—at least not for the public showed it to me several years ago a little to find ways to alleviate the at large. Bravo. and it’s been fun to watch it grow pain (aside from standard Rx’s from in quality and content over the — ANOnymOus doctors). […] years. My dad adjunctly teaches a Letter to Erowid psychopharmacology class in town and Keep up the good work. Although always lists Erowid on his syllabus of some might look at Erowid negatively, recommended readings. I’m a college I look at it positively, in the sense that After looking up information on the student and am surprised, once I I’m smart enough to research things antitussive properties of DXM, how start talking to other kids, how many before I try them, and hopefully keep shocked I was to find your website, of them know about the information myself from an early demise. -

Artemisia Californica Less
I. SPECIES Artemisia californica Less. [Updated 2017] NRCS CODE: Subtribe: Artemisiinae ARCA11 Tribe: Anthemideae (FEIS CODE: Family: Asteraceae ARCAL) Order: Asterales Subclass: Asteridae Class: Magnoliopsida flowering heads spring growth seedling, March 2009 juvenile plant photos A. Montalvo flowering plant, November 2005 mature plant with flower buds August 2010 A. Subspecific taxa None. Artemisia californica Less. var. insularis (Rydb.) Munz is now recognized as Artemisia nesiotica P.H. Raven (Jepson eFlora 2017). B. Synonyms Artemisia abrotanoides Nuttall; A. fischeriana Besser; A. foliosa Nuttall; Crossostephium californicum (Lessing) Rydberg (FNA 2017). C. Common name California sagebrush. The common name refers to its strong, sage-like aroma and endemism to California and Baja California. Other names include: coastal sage, coast sage, coast sagebrush (Painter 2016). D. Taxonomic relationships The FNA (2017) places this species in subgenus Artemisia . The molecular phylogeny of the genus has improved the understanding of relationships among the many species of Artemisia and has, at times, placed the species in subgenus Tridentadae; morphology of the inflorescences and flowers alone does not place this species with its closest relatives (Watson et al. 2002). The detailed phylogeny is not completely resolved (Hayat et al. 2009). E. Related taxa in region There are 18 species and a total of 31 taxa (including infrataxa) of Artemisia in southern California, all of which differ clearly from A. californica in habitat affinity, structure, or both (Munz 1974, Jepson eFlora 2017). Within subgenus Artemisia (as per FNA 2017), A. nesiotica from the Channel Islands is the most similar and was once considered part of A. californica ; it can be distinguished by its wider leaves with flat leaf margins (not rolled under). -

The Use of Naturopathy As Adjuvant to Traditional Medicine
The Use of Naturopathy as Adjuvant to Traditional Medicine Griffin T. Johnston1 1College of Business, Colorado State University, Campus 1052, Fort CO 80523-1052, USA Abstract: Alternative medicines have long offered opportunities for patients and doctors to treat illness. While many doctors and patients are uncomfortable with alternative medicines the use of non-traditional therapies has never been more popular. Being uncomfortable with alternative medicines can result from traditional doctors receiving little training in those fields and that patients have a difficult time finding quantifiable data on treatments resulting from the lack of funding for research into these fields1. Naturopathic medicine is one of the many forms of alternative medicine. It is a distinct primary care profession that focusses on several principles in order to care for patients. Naturopathic medicine should be promoted as an adjuvant therapy but not as a true replacement to modern medicine. BENEFITS OF NATUROPATHIC MEDICINE improvement over traditional medicine. Some Naturopathic medicine should be promoted for patients also feel more comfortable with alternative adjuvant use due to the benefits to patients that result medicine after losing faith in traditional practices. from its main principles. The principles of Most patients do not see traditional medicine as naturopathic medicine are the healing power of ineffective but as a result of a bad experience or nature, identifying and treating the causes of illness, disenchantment with it seek another option. This is a not harming patients, doctors being a teacher to further reason to support the use of naturopathic patients, treating the whole person, and finally medicine as an adjuvant therapy due to it giving prevention. -

Antileishmanial Compounds from Nature - Elucidation of the Active Principles of an Extract from Valeriana Wallichii Rhizomes
ANTILEISHMANIAL COMPOUNDS FROM NATURE - ELUCIDATION OF THE ACTIVE PRINCIPLES OF AN EXTRACT FROM VALERIANA WALLICHII RHIZOMES Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Jan Glaser aus Hammelburg Würzburg 2015 ANTILEISHMANIAL COMPOUNDS FROM NATURE - ELUCIDATION OF THE ACTIVE PRINCIPLES OF AN EXTRACT FROM VALERIANA WALLICHII RHIZOMES Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Julius-Maximilians-Universität Würzburg vorgelegt von Jan Glaser aus Hammelburg Würzburg 2015 Eingereicht am ....................................... bei der Fakultät für Chemie und Pharmazie 1. Gutachter Prof. Dr. Ulrike Holzgrabe 2. Gutachter ........................................ der Dissertation 1. Prüfer Prof. Dr. Ulrike Holzgrabe 2. Prüfer ......................................... 3. Prüfer ......................................... des öffentlichen Promotionskolloquiums Datum des öffentlichen Promotionskolloquiums .................................................. Doktorurkunde ausgehändigt am .................................................. "Wer nichts als Chemie versteht, versteht auch die nicht recht." Georg Christoph Lichtenberg (1742-1799) DANKSAGUNG Die vorliegende Arbeit wurde am Institut für Pharmazie und Lebensmittelchemie der Bayerischen Julius-Maximilians-Universität Würzburg auf Anregung und unter Anleitung von Frau Prof. Dr. Ulrike Holzgrabe und finanzieller Unterstützung der Deutschen Forschungsgemeinschaft (SFB 630) angefertigt. Ich -

Appendix G Local Plant List 2013 APPENDIX
Appendix G Local Plant List 2013 APPENDIX G LOCAL PLANT LIST PLANT LIST RECOMMENDATIONS Green Roofs Note: The following list is from the Green Roofs – Cooling Los Angeles: Resource Guide and provides vegetated roof plants applicable to Santa Barbara. For more information visit, http://www.fypower.org/pdf/LA_GreenRoofsResourceGuide.pdf. For roof garden plants, use sun and drought tolerant, self-sustaining native trees, shrubs and ecoroof plants. Common Name Scientific Name Gold Tooth Aloe Aloe nobilis Golden Barrel Cactus Echinocactus grusonii Hasse’s Dudleya Dudleya hassei Beavertail Prickly Pear Opuntia basilaris Blue-blad Cactus Opuntia violacea santa-rita Chalk Dudleya Dudleya Pulverulenta Felt Plant Kalanchoe beharensis Ice Plant Delosperma cooperii Lampranthus Lampranthus productus October Daphne Sedum sieboldii Oscularia Lampranthus deltoids Purple Stonecrop Sedum spathulifolium White Trailing Ice Plant Delosperma Alba Brown Sedge Carex testacea Deer Grass Muhlenbergia rigens Tussock Sedge Carex stricta Many species of agave Bioretention Areas, Rain Gardens, Planter Boxes, Infiltration Basins, Vegetated Swales, Vegetated Filter Strips, and Dry Extended Detention Basins: The plants listed in this section include native plantings that are suitable for areas that will receive short periods of inundation (e.g. 24 to 72 hours) as well as plants suitable for upland areas. Native Plantings – Trees (Can Handle Short Periods of Inundation) Common Name Scientific Name Western Sycamore Platanus racemosa Freemont Cottonwood Populus fremontii -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

The Advantages of Traditional Chumash Healing
eCAM 2005;2(1)19–23 doi:10.1093/ecam/neh072 Lecture Series The Advantages of Traditional Chumash Healing James D. Adams Jr1 and Cecilia Garcia2 1Department of Molecular Pharmacology and Toxicology, University of Southern California, School of Pharmacy, 1985 Zonal Avenue, PSC 508, Los Angeles and 2Chumash Healer, Granada Hills, CA, USA Chumash healing has been practiced in California for ~13 000 years. Chumash healers treat their patients with prayer, laughter, dreaming, phytotherapy, aromatherapy, healing ceremonies and other techniques. Healing involves first healing the spirit, then healing the body. Chumash people still main- tain their unique identity. Chumash Healers still practice the ancient healing arts in California. This lecture is a brief introduction to Chumash Healing. Keywords: healing – ethnopharmacology – Chumash Introduction prayer has been shown to be beneficial to the majority of patients (1). Prayer comforts patients. At the very least, prayer Traditional Chumash healing starts with God. The Healer may does no harm and costs nothing. While in hospital, the patient use prayer and fasting to invite God to help with the healing. may feel isolated from family and friends. The spirit may not Fasting is a self-denial that enhances the spirituality of the become well quickly. The approach with standard health care Healer, facilitating communication with God. In fact, all healing is that the body is treated and made well. The spirit should then must involve God. The spirit is always involved in the healing follow and recover as well. However, many patients complain process. The reason for this is that many diseases start with of uncertainty with their healing even after their bodies are the spirit. -

Plant Breading
SNA Research Conference Vol. 52 2007 Plant Breeding and Evaluation Tom Ranney Section Editor and Moderator Plant Breeding and Evaluation Section 326 SNA Research Conference Vol. 52 2007 New Callicarpa Species with Breeding Potential Ryan N. Contreras and John M. Ruter University of Georgia, Dept. of Horticulture, Tifton, GA 31793 [email protected] Index Words: beautyberry, species evaluation, ornamental plant breeding Significance to Industry: There is a great deal of available Callicarpa L. germplasm that has yet to be utilized by the nursery industry in the U.S. Taxa currently being evaluated are likely to have potential as breeding material or direct commercial marketability. With new breeding material and selections for introduction the number of beautyberry cultivars for use in southeastern gardens has the potential to expand greatly. Nature of Work: Callicarpa L. is a genus of ~150 species of shrubs and trees distributed throughout the world including warm-temperate and tropical America, SE Asia, Malaysia, Pacific Islands, and Australia (5) with the greatest concentration of species found in SE Asia, specifically the Philippine Islands (1). Of the New World species the highest concentration occurs in Cuba, with ~20 native species (1). There are currently four species commonly found in cultivation in the U.S.: C. americana L., C. bodinieri Lév., C. dichotoma (Lour.)K.Koch, and C. japonica Thunb. with a limited number of varieties or cultivars of each to choose from (3). Beautyberries, desired primarily for their handsome berries produced in fall, have been selected for white-fruiting varieties, finer textured varieties, increased berry production, and variegated foliage.