Integration Drives Rapid Phenotypic Evolution in Flatfishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FISH LIST WISH LIST: a Case for Updating the Canadian Government’S Guidance for Common Names on Seafood
FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood Authors: Christina Callegari, Scott Wallace, Sarah Foster and Liane Arness ISBN: 978-1-988424-60-6 © SeaChoice November 2020 TABLE OF CONTENTS GLOSSARY . 3 EXECUTIVE SUMMARY . 4 Findings . 5 Recommendations . 6 INTRODUCTION . 7 APPROACH . 8 Identification of Canadian-caught species . 9 Data processing . 9 REPORT STRUCTURE . 10 SECTION A: COMMON AND OVERLAPPING NAMES . 10 Introduction . 10 Methodology . 10 Results . 11 Snapper/rockfish/Pacific snapper/rosefish/redfish . 12 Sole/flounder . 14 Shrimp/prawn . 15 Shark/dogfish . 15 Why it matters . 15 Recommendations . 16 SECTION B: CANADIAN-CAUGHT SPECIES OF HIGHEST CONCERN . 17 Introduction . 17 Methodology . 18 Results . 20 Commonly mislabelled species . 20 Species with sustainability concerns . 21 Species linked to human health concerns . 23 Species listed under the U .S . Seafood Import Monitoring Program . 25 Combined impact assessment . 26 Why it matters . 28 Recommendations . 28 SECTION C: MISSING SPECIES, MISSING ENGLISH AND FRENCH COMMON NAMES AND GENUS-LEVEL ENTRIES . 31 Introduction . 31 Missing species and outdated scientific names . 31 Scientific names without English or French CFIA common names . 32 Genus-level entries . 33 Why it matters . 34 Recommendations . 34 CONCLUSION . 35 REFERENCES . 36 APPENDIX . 39 Appendix A . 39 Appendix B . 39 FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood 2 GLOSSARY The terms below are defined to aid in comprehension of this report. Common name — Although species are given a standard Scientific name — The taxonomic (Latin) name for a species. common name that is readily used by the scientific In nomenclature, every scientific name consists of two parts, community, industry has adopted other widely used names the genus and the specific epithet, which is used to identify for species sold in the marketplace. -

Reef Fish Biodiversity in the Florida Keys National Marine Sanctuary Megan E
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School November 2017 Reef Fish Biodiversity in the Florida Keys National Marine Sanctuary Megan E. Hepner University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Biology Commons, Ecology and Evolutionary Biology Commons, and the Other Oceanography and Atmospheric Sciences and Meteorology Commons Scholar Commons Citation Hepner, Megan E., "Reef Fish Biodiversity in the Florida Keys National Marine Sanctuary" (2017). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/7408 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Reef Fish Biodiversity in the Florida Keys National Marine Sanctuary by Megan E. Hepner A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Marine Science with a concentration in Marine Resource Assessment College of Marine Science University of South Florida Major Professor: Frank Muller-Karger, Ph.D. Christopher Stallings, Ph.D. Steve Gittings, Ph.D. Date of Approval: October 31st, 2017 Keywords: Species richness, biodiversity, functional diversity, species traits Copyright © 2017, Megan E. Hepner ACKNOWLEDGMENTS I am indebted to my major advisor, Dr. Frank Muller-Karger, who provided opportunities for me to strengthen my skills as a researcher on research cruises, dive surveys, and in the laboratory, and as a communicator through oral and presentations at conferences, and for encouraging my participation as a full team member in various meetings of the Marine Biodiversity Observation Network (MBON) and other science meetings. -

A Practical Handbook for Determining the Ages of Gulf of Mexico And
A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes THIRD EDITION GSMFC No. 300 NOVEMBER 2020 i Gulf States Marine Fisheries Commission Commissioners and Proxies ALABAMA Senator R.L. “Bret” Allain, II Chris Blankenship, Commissioner State Senator District 21 Alabama Department of Conservation Franklin, Louisiana and Natural Resources John Roussel Montgomery, Alabama Zachary, Louisiana Representative Chris Pringle Mobile, Alabama MISSISSIPPI Chris Nelson Joe Spraggins, Executive Director Bon Secour Fisheries, Inc. Mississippi Department of Marine Bon Secour, Alabama Resources Biloxi, Mississippi FLORIDA Read Hendon Eric Sutton, Executive Director USM/Gulf Coast Research Laboratory Florida Fish and Wildlife Ocean Springs, Mississippi Conservation Commission Tallahassee, Florida TEXAS Representative Jay Trumbull Carter Smith, Executive Director Tallahassee, Florida Texas Parks and Wildlife Department Austin, Texas LOUISIANA Doug Boyd Jack Montoucet, Secretary Boerne, Texas Louisiana Department of Wildlife and Fisheries Baton Rouge, Louisiana GSMFC Staff ASMFC Staff Mr. David M. Donaldson Mr. Bob Beal Executive Director Executive Director Mr. Steven J. VanderKooy Mr. Jeffrey Kipp IJF Program Coordinator Stock Assessment Scientist Ms. Debora McIntyre Dr. Kristen Anstead IJF Staff Assistant Fisheries Scientist ii A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes Third Edition Edited by Steve VanderKooy Jessica Carroll Scott Elzey Jessica Gilmore Jeffrey Kipp Gulf States Marine Fisheries Commission 2404 Government St Ocean Springs, MS 39564 and Atlantic States Marine Fisheries Commission 1050 N. Highland Street Suite 200 A-N Arlington, VA 22201 Publication Number 300 November 2020 A publication of the Gulf States Marine Fisheries Commission pursuant to National Oceanic and Atmospheric Administration Award Number NA15NMF4070076 and NA15NMF4720399. -
Schedule Onlinepdf
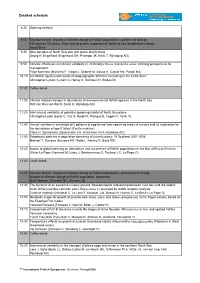
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Collapse and Recovery of Marine Fishes
letters to nature sulphide complexes, indicating that Cu sulphide clusters are more 7. Al-Farawati, R. & van den Berg, C. M. G. Metal-sulfide complexation in seawater. Mar. Chem. 63, 331–352 (1999). stable than Cu organic complexes. This explains why laboratory 8. Luther III, G. W., Rickard, D. T., Theberge, S. M. & Olroyd, A. Determination of metal (bi)sulfide cultures of oceanic phytoplankton have been observed to increase stability constants of Mn2+,Fe2+,Co2+,Ni2+,Cu2+, and Zn2+ by voltammetric methods. Environ. Sci. the production of total dissolved sulphides when the concentrations Technol. 30, 671–679 (1996). of free Cu and Zn in the culture media were increased22. Although 9. Helz, G. R, Charnock, J. M., Vaughan, D. J. & Garner, C. D. Multinuclearity of aqueous copper and zinc bisulfide complexes—an EXAFS investigation. Geochim. Cosmochim. Acta 57, the data that we report here suggest that metal sulphide formation is 15–25 (1993). a means of detoxifying trace metals for organisms, further toxico- 10. Luther III, G. W., Theberge, S. M. & Rickard, D. T. Evidence for aqueous clusters as intermediates logical studies are needed to quantify the roles both sulphides and during zinc sulfide formation. Geochim. Cosmochim. Acta 19/20, 3159–3169 (1999). ‘natural’ organic ligands play in controlling Cu toxicity in natural 11. Peters, J. W., Lanzilotta, W. N., Lemon, B. J. & Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (Cpl) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853– waters. 1858 (1998). Sulphur complexation may have a dramatic effect on the acute 12. -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

Why Are There So Many Flatfishes? Jaw Asymmetry, Diet, and Diversification in the Pleuronectiformes
Why are there so many flatfishes? Jaw asymmetry, diet, and diversification in the Pleuronectiformes Jonathan Chang1 Functional Morphology and Ecology of Fishes Summer 2014 1Department of Ecology and Evolutionary Biology, University of California, Los Angeles, CA 90095, USA. Contact information: Jonathan Chang 610 Charles E. Young Drive S Los Angeles CA 90095 [email protected] Keywords: flatfish, Pleuronectidae, Paralichthyidae, Bothidae, asymmetry, geometric morphometrics, comparative methods, functional morphology Chang 1 Abstract Flatfishes (Actinopterygii: Pleuronectiformes) are a diverse group of teleost fishes, with over 700 species in the order. Jaw asymmetry and diet have been thought to contribute to flatfish diversity but this has not yet been tested in a comparative framework. Here I use geometric morphometric and comparative methods to test whether ocular-blind side asymmetry in flatfish head morphology contributed to flatfish diversification. I find that the repeated convergent evolution of similar morphology, jaw function, and diet likely contribute to the high diversity of flatfishes. Introduction Pleuronectiform fishes are highly diverse, with over 700 described species (Froese and Pauly, 2014). These fishes are characterized by their unique bilateral asymmetry and their benthic ecology. Flatfishes also generally consume one of three main types of prey: buried infauna, pelagic fishes and crustaceans, and a third type intermediate to the first two (de Groot 1971, Tsuruta & Omori 1976). I hypothesize that this specialization into different prey types has driven the diversification and morphological disparity in asymmetry of flatfish species. Methods 12 species of flatfish comprising of 11 genera and 2 families (Table 1) were collected via trawl and seine at these sites: Jackson Beach, [48°31'13.0"N 123°00'35.1"W] and Orcas – Eastsound [48°38'26.9"N 122°52'14.0"W]. -

Pleuronectidae
FAMILY Pleuronectidae Rafinesque, 1815 - righteye flounders [=Heterosomes, Pleronetti, Pleuronectia, Diplochiria, Poissons plats, Leptosomata, Diprosopa, Asymmetrici, Platessoideae, Hippoglossoidinae, Psettichthyini, Isopsettini] Notes: Hétérosomes Duméril, 1805:132 [ref. 1151] (family) ? Pleuronectes [latinized to Heterosomi by Jarocki 1822:133, 284 [ref. 4984]; no stem of the type genus, not available, Article 11.7.1.1] Pleronetti Rafinesque, 1810b:14 [ref. 3595] (ordine) ? Pleuronectes [published not in latinized form before 1900; not available, Article 11.7.2] Pleuronectia Rafinesque, 1815:83 [ref. 3584] (family) Pleuronectes [senior objective synonym of Platessoideae Richardson, 1836; family name sometimes seen as Pleuronectiidae] Diplochiria Rafinesque, 1815:83 [ref. 3584] (subfamily) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Poissons plats Cuvier, 1816:218 [ref. 993] (family) Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Leptosomata Goldfuss, 1820:VIII, 72 [ref. 1829] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Diprosopa Latreille, 1825:126 [ref. 31889] (family) Platessa [no stem of the type genus, not available, Article 11.7.1.1] Asymmetrici Minding, 1832:VI, 89 [ref. 3022] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Platessoideae Richardson, 1836:255 [ref. 3731] (family) Platessa [junior objective synonym of Pleuronectia Rafinesque, 1815, invalid, Article 61.3.2 Hippoglossoidinae Cooper & Chapleau, 1998:696, 706 [ref. 26711] (subfamily) Hippoglossoides Psettichthyini Cooper & Chapleau, 1998:708 [ref. 26711] (tribe) Psettichthys Isopsettini Cooper & Chapleau, 1998:709 [ref. 26711] (tribe) Isopsetta SUBFAMILY Atheresthinae Vinnikov et al., 2018 - righteye flounders GENUS Atheresthes Jordan & Gilbert, 1880 - righteye flounders [=Atheresthes Jordan [D. -

Field Manual for Coral Reef Assessments
EPA/600/R-12/029 | April 2012 |www.epa.gov/ged Field Manual for Coral Reef Assessments Office of Research and Development National Health and Environmental Effects Research Laboratory Gulf Ecology Division Field Manual for Coral Reef Assessments Deborah L. Santavy William S. Fisher Jed G. Campbell Robert L. Quarles Gulf Ecology Division National Health and Environmental Effects Research Laboratory Office of Research and Development U.S. Environmental Protection Agency 1 Sabine Island Dr. Gulf Breeze, FL. 32561 Notice and Disclaimer The U.S. Environmental Protection Agency through its Office of Research and Development and Office of Water funded and collaborated in the research and development of these field protocols. It has been subjected to the Agency’s peer and administrative review and has been approved for publication as an EPA document. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This is a contribution to the EPA Office of Research and Development’s Safe and Sustainable Water Resources Program, Coral Reefs Project. The appropriate citation for this report is: Santavy DL, Fisher WS, Campbell JG and Quarles RL. 2012. Field Manual for Coral Reef Assessments. U.S. Environmental Protection Agency, Office of Research and Development, Gulf Ecology Division, Gulf Breeze, FL. EPA/600/R-12/029. April 2012. This document can be downloaded from EPA’s website at: http://www.epa.gov/ged/publications.html ii Table of Contents Notice and Disclaimer ................................................................................................................................ -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

Offshore Marine Actinopterygian Assemblages from the Maastrichtian–Paleogene of the Pindos Unit in Eurytania, Greece Thodoris Argyriou, Donald Davesne
Offshore marine actinopterygian assemblages from the Maastrichtian–Paleogene of the Pindos Unit in Eurytania, Greece Thodoris Argyriou, Donald Davesne To cite this version: Thodoris Argyriou, Donald Davesne. Offshore marine actinopterygian assemblages from the Maastrichtian–Paleogene of the Pindos Unit in Eurytania, Greece. PeerJ, PeerJ, 2021, 9, pp.e10676. 10.7717/peerj.10676. hal-03121049 HAL Id: hal-03121049 https://hal.sorbonne-universite.fr/hal-03121049 Submitted on 26 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Offshore marine actinopterygian assemblages from the Maastrichtian–Paleogene of the Pindos Unit in Eurytania, Greece Thodoris Argyriou1 and Donald Davesne2,3 1 UMR 7207 (MNHN—Sorbonne Université—CNRS) Centre de Recherche en Paléontologie, Museum National d’Histoire naturelle, Paris, France 2 Department of Earth Sciences, University of Oxford, Oxford, UK 3 UMR 7205 (MNHN—Sorbonne Université—CNRS—EPHE), Institut de Systématique, Évolution, Biodiversité, Museum National d’Histoire naturelle, Paris, France ABSTRACT The fossil record of marine ray-finned fishes (Actinopterygii) from the time interval surrounding the Cretaceous–Paleogene (K–Pg) extinction is scarce at a global scale, hampering our understanding of the impact, patterns and processes of extinction and recovery in the marine realm, and its role in the evolution of modern marine ichthyofaunas. -

Yellowfin Trawling Fish Images 2013 09 16
Fishes captured aboard the RV Yellowfin in otter trawls: September 2013 Order: Aulopiformes Family: Synodontidae Species: Synodus lucioceps common name: California lizardfish Order: Gadiformes Family: Merlucciidae Species: Merluccius productus common name: Pacific hake Order: Ophidiiformes Family: Ophidiidae Species: Chilara taylori common name: spotted cusk-eel plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman Order: Gasterosteiformes Family: Syngnathidae Species: Syngnathus leptorynchus common name: bay pipefish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes semicinctus common name: halfbanded rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes dalli common name: calico rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes saxicola common name: stripetail rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes diploproa common name: splitnose rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes rosenblatti common name: greenblotched rockfish juvenile Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes levis common name: cowcod Order: