Variation in the Nicotinic Acetylcholine Receptor Gene Cluster CHRNA5–CHRNA3–CHRNB4 and Its Interaction with Recent Tobacco Use Influence Cognitive Flexibility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Article Microarray-Based Comparisons of Ion Channel Expression Patterns: Human Keratinocytes to Reprogrammed Hipscs To
Hindawi Publishing Corporation Stem Cells International Volume 2013, Article ID 784629, 25 pages http://dx.doi.org/10.1155/2013/784629 Research Article Microarray-Based Comparisons of Ion Channel Expression Patterns: Human Keratinocytes to Reprogrammed hiPSCs to Differentiated Neuronal and Cardiac Progeny Leonhard Linta,1 Marianne Stockmann,1 Qiong Lin,2 André Lechel,3 Christian Proepper,1 Tobias M. Boeckers,1 Alexander Kleger,3 and Stefan Liebau1 1 InstituteforAnatomyCellBiology,UlmUniversity,Albert-EinsteinAllee11,89081Ulm,Germany 2 Institute for Biomedical Engineering, Department of Cell Biology, RWTH Aachen, Pauwelstrasse 30, 52074 Aachen, Germany 3 Department of Internal Medicine I, Ulm University, Albert-Einstein Allee 11, 89081 Ulm, Germany Correspondence should be addressed to Alexander Kleger; [email protected] and Stefan Liebau; [email protected] Received 31 January 2013; Accepted 6 March 2013 Academic Editor: Michael Levin Copyright © 2013 Leonhard Linta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Ion channels are involved in a large variety of cellular processes including stem cell differentiation. Numerous families of ion channels are present in the organism which can be distinguished by means of, for example, ion selectivity, gating mechanism, composition, or cell biological function. To characterize the distinct expression of this group of ion channels we have compared the mRNA expression levels of ion channel genes between human keratinocyte-derived induced pluripotent stem cells (hiPSCs) and their somatic cell source, keratinocytes from plucked human hair. This comparison revealed that 26% of the analyzed probes showed an upregulation of ion channels in hiPSCs while just 6% were downregulated. -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -

Replicated Risk Nicotinic Cholinergic Receptor Genes for Nicotine Dependence
G C A T T A C G G C A T genes Article Replicated Risk Nicotinic Cholinergic Receptor Genes for Nicotine Dependence Lingjun Zuo 1, Rolando Garcia-Milian 2, Xiaoyun Guo 1,3,4,*, Chunlong Zhong 5,*, Yunlong Tan 6, Zhiren Wang 6, Jijun Wang 3, Xiaoping Wang 7, Longli Kang 8, Lu Lu 9,10, Xiangning Chen 11,12, Chiang-Shan R. Li 1 and Xingguang Luo 1,6,* 1 Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06510, USA; [email protected] (L.Z.); [email protected] (C.-S.R.L.) 2 Curriculum & Research Support Department, Cushing/Whitney Medical Library, Yale University School of Medicine, New Haven, CT 06510, USA; [email protected] 3 Shanghai Mental Health Center, Shanghai 200030, China; [email protected] 4 Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06510, USA 5 Department of Neurosurgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China 6 Biological Psychiatry Research Center, Beijing Huilongguan Hospital, Beijing 100096, China; [email protected] (Y.T.); [email protected] (Z.W.) 7 Department of Neurology, Shanghai First People’s Hospital, Shanghai Jiao Tong University, Shanghai 200080, China; [email protected] 8 Key Laboratory for Molecular Genetic Mechanisms and Intervention Research on High Altitude Diseases of Tibet Autonomous Region, Xizang Minzu University School of Medicine, Xianyang, Shanxi 712082, China; [email protected] 9 Provincial Key Laboratory for Inflammation and Molecular Drug Target, Medical -

Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology
brain sciences Review Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology Andrea Becchetti 1,* , Laura Clara Grandi 1 , Giulia Colombo 1 , Simone Meneghini 1 and Alida Amadeo 2 1 Department of Biotechnology and Biosciences, University of Milano-Bicocca, 20126 Milano, Italy; [email protected] (L.C.G.); [email protected] (G.C.); [email protected] (S.M.) 2 Department of Biosciences, University of Milano, 20133 Milano, Italy; [email protected] * Correspondence: [email protected] Received: 13 October 2020; Accepted: 21 November 2020; Published: 25 November 2020 Abstract: Sleep-related hypermotor epilepsy (SHE) is characterized by hyperkinetic focal seizures, mainly arising in the neocortex during non-rapid eye movements (NREM) sleep. The familial form is autosomal dominant SHE (ADSHE), which can be caused by mutations in genes encoding subunits of the neuronal nicotinic acetylcholine receptor (nAChR), Na+-gated K+ channels, as well as non-channel signaling proteins, such as components of the gap activity toward rags 1 (GATOR1) macromolecular complex. The causative genes may have different roles in developing and mature brains. Under this respect, nicotinic receptors are paradigmatic, as different pathophysiological roles are exerted by distinct nAChR subunits in adult and developing brains. The widest evidence concerns α4 and β2 subunits. These participate in heteromeric nAChRs that are major modulators of excitability in mature neocortical circuits as well as regulate postnatal synaptogenesis. However, growing evidence implicates mutant α2 subunits in ADSHE, which poses interpretive difficulties as very little is known about the function of α2-containing (α2*) nAChRs in the human brain. -

Resequencing of Nicotinic Acetylcholine Receptor Genes and Association of Common and Rare Variants with the Fagerstro¨M Test for Nicotine Dependence
Neuropsychopharmacology (2010) 35, 2392–2402 & 2010 Nature Publishing Group All rights reserved 0893-133X/10 $32.00 www.neuropsychopharmacology.org Resequencing of Nicotinic Acetylcholine Receptor Genes and Association of Common and Rare Variants with the Fagerstro¨m Test for Nicotine Dependence 1,4 1 2 2 1 Jennifer Wessel , Sarah M McDonald , David A Hinds , Renee P Stokowski , Harold S Javitz , 2 1 2 1 2 1 Michael Kennemer , Ruth Krasnow , William Dirks , Jill Hardin , Steven J Pitts , Martha Michel , 1 2 3 1 ,1 Lisa Jack , Dennis G Ballinger , Jennifer B McClure , Gary E Swan and Andrew W Bergen* 1 2 3 Center for Health Sciences, SRI International, Menlo Park, CA, USA; Perlegen Sciences, Mountain View, CA, USA; Group Health Research 4 Institute, Seattle, WA, USA; Department of Public Health, Indiana University School of Medicine, Indianapolis, IN, USA Common single-nucleotide polymorphisms (SNPs) at nicotinic acetylcholine receptor (nAChR) subunit genes have previously been associated with measures of nicotine dependence. We investigated the contribution of common SNPs and rare single-nucleotide variants (SNVs) in nAChR genes to Fagerstro¨m test for nicotine dependence (FTND) scores in treatment-seeking smokers. Exons of 10 genes were resequenced with next-generation sequencing technology in 448 European-American participants of a smoking cessation trial, and CHRNB2 and CHRNA4 were resequenced by Sanger technology to improve sequence coverage. A total of 214 SNP/SNVs were identified, of which 19.2% were excluded from analyses because of reduced completion rate, 73.9% had minor allele frequencies o5%, and 48.1% were novel relative to dbSNP build 129. -

Retrograde Inhibition by a Specific Subset of Interpeduncular Α5 Nicotinic Neurons Regulates Nicotine Preference
Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference Jessica L. Ablesa,b,c, Andreas Görlicha,1, Beatriz Antolin-Fontesa,2,CuidongWanga, Sylvia M. Lipforda, Michael H. Riada, Jing Rend,e,3,FeiHud,e,4,MinminLuod,e,PaulJ.Kennyc, Nathaniel Heintza,f,5, and Ines Ibañez-Tallona,5 aLaboratory of Molecular Biology, The Rockefeller University, New York, NY 10065; bDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY 10029; cDepartment of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY 10029; dNational Institute of Biological Sciences, Beijing 102206, China; eSchool of Life Sciences, Tsinghua University, Beijing 100084, China; and fHoward Hughes Medical Institute, The Rockefeller University, New York, NY 10065 Contributed by Nathaniel Heintz, October 23, 2017 (sent for review October 5, 2017; reviewed by Jean-Pierre Changeux and Lorna W. Role) Repeated exposure to drugs of abuse can produce adaptive changes nicotine withdrawal, and optical activation of IPN GABAergic cells that lead to the establishment of dependence. It has been shown that is sufficient to produce a withdrawal syndrome, while blockade of allelic variation in the α5 nicotinic acetylcholine receptor (nAChR) gene GABAergic cells in the IPN reduced symptoms of withdrawal (17). CHRNA5 is associated with higher risk of tobacco dependence. In the Taken together these studies highlight the critical role of α5in brain, α5-containing nAChRs are expressed at very high levels in the regulating behavioral responses to nicotine. Here we characterize two subpopulations of GABAergic interpeduncular nucleus (IPN). Here we identified two nonoverlapping Amigo1 Epyc α + α Amigo1 α Epyc neurons in the IPN that express α5: α5- and α5- neu- 5 cell populations ( 5- and 5- ) in mouse IPN that respond α Amigo1 α Epyc differentially to nicotine. -

Retrograde Inhibition by a Specific Subset of Interpeduncular Α5 Nicotinic Neurons Regulates Nicotine Preference
Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference Jessica L. Ablesa,b,c, Andreas Görlicha,1, Beatriz Antolin-Fontesa,2,CuidongWanga, Sylvia M. Lipforda, Michael H. Riada, Jing Rend,e,3,FeiHud,e,4,MinminLuod,e,PaulJ.Kennyc, Nathaniel Heintza,f,5, and Ines Ibañez-Tallona,5 aLaboratory of Molecular Biology, The Rockefeller University, New York, NY 10065; bDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY 10029; cDepartment of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY 10029; dNational Institute of Biological Sciences, Beijing 102206, China; eSchool of Life Sciences, Tsinghua University, Beijing 100084, China; and fHoward Hughes Medical Institute, The Rockefeller University, New York, NY 10065 Contributed by Nathaniel Heintz, October 23, 2017 (sent for review October 5, 2017; reviewed by Jean-Pierre Changeux and Lorna W. Role) Repeated exposure to drugs of abuse can produce adaptive changes nicotine withdrawal, and optical activation of IPN GABAergic cells that lead to the establishment of dependence. It has been shown that is sufficient to produce a withdrawal syndrome, while blockade of allelic variation in the α5 nicotinic acetylcholine receptor (nAChR) gene GABAergic cells in the IPN reduced symptoms of withdrawal (17). CHRNA5 is associated with higher risk of tobacco dependence. In the Taken together these studies highlight the critical role of α5in brain, α5-containing nAChRs are expressed at very high levels in the regulating behavioral responses to nicotine. Here we characterize two subpopulations of GABAergic interpeduncular nucleus (IPN). Here we identified two nonoverlapping Amigo1 Epyc α + α Amigo1 α Epyc neurons in the IPN that express α5: α5- and α5- neu- 5 cell populations ( 5- and 5- ) in mouse IPN that respond α Amigo1 α Epyc differentially to nicotine. -
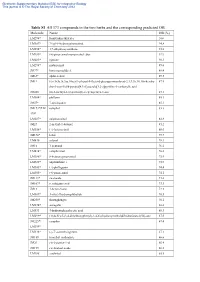
Table S1 All 373 Compounds in the Two Herbs and the Corresponding
Electronic Supplementary Material (ESI) for Integrative Biology This journal is © The Royal Society of Chemistry 2012 Table S1 All 373 compounds in the two herbs and the corresponding predicted OB Molecule Name OB (%) LM294* forsythidmethylester 100 LM387* 7'-epi-8-hydroxypinoresinol 94.8 LM394* 1,7-dihydroxyxanthone 93.8 LM289* (+)-pinoresinol monomethyl ether 92.9 LM285* egenine 90.3 LM298* matairesinol 89.6 JM77* loniceracetalide A 89.4 JM63* alpha-cedrol 89.3 JM1* (-)-(3r,8s,9r,9as,10as)-9-ethenyl-8-(beta-d-glucopyranosyloxy)-2,3,9,9a,10,10a-hexahy 87.5 dro-5-oxo-5h,8h-pyrano[4,3-d]oxazolo[3,2-a]pyridine-3-carboxylic acid JM200 (z)-3-methyl-2-(2-pentenyl)-2-cyclopenten-1-one 87.1 LM304* phillyrin 85.1 JM57* 7-epi-loganin 85.1 JM173*/LM camphol 83.3 398* LM287* epipinoresinol 82.8 JM27 2-methyl-1-butanol 81.2 LM356* (+)-lariciresinol 80.0 JM170* ledol 79.7 LM430 safynol 78.3 JM16 1-pentanol 76.2 LM414* campherenol 76.0 LM386* 8-hydroxypinoresinol 75.9 LM428* onjixanthone i 75.9 LM345* (+)-phillygenin 74.4 LM305* (+)-pinoresinol 74.1 JM113* sweroside 73.6 JM107* secologanic acid 73.3 JM13 1-hexen-3-one 72.3 LM309* 3-ethyl-7hydroxyphthalide 70.5 JM250* shuangkangsu 70.1 LM274* astragalin 68.6 LM331 4-hydroxyphenylacetic acid 68.3 LM299* (3r,4s,5r)-5-(3,4-dimethoxyphenyl)-3,4-bis(hydroxymethyl)dihydrofuran-2(3h)-one 67.5 JM225*/ camphor 67.4 LM399* LM338* (-)-7’-o-methylegenine 67.1 JM189 trimethyl isothiazole 66.6 JM29 cis-2-penten-1-ol 66.4 JM199 cis-linalool oxide 66.2 LM288 erythritol 65.8 Electronic Supplementary Material (ESI) for -

Neural Circuits and Nicotinic Acetylcholine Receptors Mediate the Cholinergic Regulation of Midbrain Dopaminergic Neurons and Nicotine Dependence
www.nature.com/aps REVIEW ARTICLE OPEN Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence Cheng Xiao1,2, Chun-yi Zhou1,2, Jin-hong Jiang1,2 and Cui Yin1,2 Midbrain dopaminergic (DA) neurons are governed by an endogenous cholinergic system, originated in the mesopontine nuclei. Nicotine hijacks nicotinic acetylcholine receptors (nAChRs) and interferes with physiological function of the cholinergic system. In this review, we describe the anatomical organization of the cholinergic system and the key nAChR subtypes mediating cholinergic regulation of DA transmission and nicotine reward and dependence, in an effort to identify potential targets for smoking intervention. Cholinergic modulation of midbrain DA systems relies on topographic organization of mesopontine cholinergic projections, and activation of nAChRs in midbrain DA neurons. Previous studies have revealed that α4, α6, and β2 subunit- containing nAChRs expressed in midbrain DA neurons and their terminals in the striatum regulate firings of midbrain DA neurons and activity-dependent dopamine release in the striatum. These nAChRs undergo modification upon chronic nicotine exposure. Clinical investigation has demonstrated that partial agonists of these receptors elevate the success rate of smoking cessation relative to placebo. However, further investigations are required to refine the drug targets to mitigate unpleasant side-effects. Keywords: midbrain DA neurons; mesopontine; cholinergic neurons; nicotinic acetylcholine receptors; neural circuits; nicotine reward and dependence; smoking intervention Acta Pharmacologica Sinica (2020) 41:1–9; https://doi.org/10.1038/s41401-019-0299-4 INTRODUCTION modified by chronic exposure to nicotine, and the subtypes of Cigarette smoking causes the most preventable diseases nAChRs implicated in nicotine dependence. -

An Observational and Genome-Wide by Environment Interaction Study in UK Biobank
Translational Psychiatry www.nature.com/tp ARTICLE OPEN Traumatic events during childhood and its risks to substance use in adulthood: an observational and genome-wide by environment interaction study in UK Biobank 1,2 1,2 1 1 1 1 1 1 1 Shiqiang Cheng , Yan Wen , Li Liu✉ , Bolun Cheng , Chujun Liang , Jing Ye , Xiaomeng Chu , Yao Yao , Yumeng Jia , Om Prakash Kafle1 and Feng Zhang 1 © The Author(s) 2021 We aimed to explore the underlying genetic mechanisms of traumatic events during childhood affecting the risks of adult substance use in present study. Using UK Biobank cohort, linear regression model was first applied to assess the relationships between cigarette smoking and alcohol drinking in adults with traumatic events during childhood, including felt hated by family member (41,648–111,465), felt loved (46,394–124,481) and sexually molested (47,598–127,766). Using traumatic events as exposure variables, genome-wide by environment interaction study was then performed by PLINK 2.0 to identify cigarette smoking and alcohol drinking associated genes interacting with traumatic events during childhood. We found that the frequency of cigarette smoking was significantly associated with felt hated by family member (coefficient = 0.42, P < 1.0 × 10–9), felt loved (coefficient = −0.31, P < 1.0 × 10–9) and sexually molested (coefficient = 0.46, P < 1.0 × 10–9). We also observed weaker associations of alcohol drinking with felt hated by family member (coefficient = 0.08, P = 3.10 × 10–6) and felt loved (coefficient = −0.06, P = 3.15 × 10–7). GWEIS identified multiple candidate loci interacting with traumatic events, such as CTNNA3 (rs189142060, P = 4.23 × 10–8) between felt hated by family member and the frequency of cigarette smoking, GABRG3 (rs117020886, P = 2.77 × 10–8) between felt hated by family member and the frequency of alcohol drinking. -
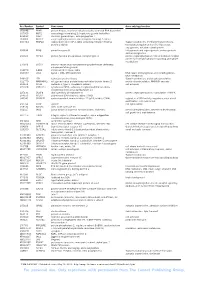
For Personal Use. Only Reproduce with Permission from the Lancet Publishing Group
Acc Number Symbol Gene name Gene ontology function U50648 PRKR protein kinase, interferon-inducible double stranded RNA dependent U37055 MST1 macrophage stimulating 1 (hepatocyte growth factor-like) K03183 CGB7 chorionic gonadotropin, beta polypeptide 7 J03069 MYCL2 v-myc myelocytomatosis viral oncogene homolog 2 (avian) M36711 TFAP2A transcription factor AP-2 alpha (activating enhancer binding Signal transduction, developmental processes, protein 2 alpha) transcription regulation from Pol II promoter, oncogenesis, ectoderm development X69699 PAX8 paired box gene 8 histogenesis and organogenesis, embryogenesis and morphogenesis L38929 PTPRD protein tyrosine phosphatase, receptor type, D protein dephosphorylation, transmembrane receptor protein tyrosine phosphatase signalling, phosphate metabolism L76568 ERCC4 excision repair cross-complementing rodent repair deficiency, complementation group 4 U11870 IL8RA interleukin 8 receptor, alpha M36067 LIG1 ligase I, DNA, ATP-dependent DNA repair, embryogenesis and morphogenesis, DNA metabolism D16105 LTK leukocyte tyrosine kinase Signal transduction, protein phosphorylation U12779 MAPKAPK2 mitogen-activated protein kinase-activated protein kinase 2 protein phosphorylation, MAPKKK cascade L34059 CDH4 cadherin 4, type 1, R-cadherin (retinal) cell adhesion U22028 CYP2A13 cytochrome P450, subfamily IIA (phenobarbital-inducible), complementation group 4polypeptide 13 U27193 DUSP8 dual specificity phosphatase 8 protein dephosphorylation, inactiviation of MAPK L24559 POLA2 polymerase (DNA-directed), alpha -

ION CHANNEL RECEPTORS TÁMOP-4.1.2-08/1/A-2009-0011 Ion Channel Receptors
Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4.1.2-08/1/A-2009-0011 Manifestation of Novel Social Challenges of the European Union in the Teaching Material of Medical Biotechnology Master’s Programmes at the University of Pécs and at the University of Debrecen Identification number: TÁMOP-4.1.2-08/1/A-2009-0011 Tímea Berki and Ferenc Boldizsár Signal transduction ION CHANNEL RECEPTORS TÁMOP-4.1.2-08/1/A-2009-0011 Ion channel receptors 1 Cys-loop receptors: pentameric structure, 4 transmembrane (TM) regions/subunit – Acetylcholin (Ach) Nicotinic R – Na+ channel - – GABAA, GABAC, Glycine – Cl channels (inhibitory role in CNS) 2 Glutamate-activated cationic channels: (excitatory role in CNS), tetrameric stucture, 3 TM regions/subunit – eg. iGlu 3 ATP-gated channels: 3 homologous subunits, 2 TM regions/subunit – eg. P2X purinoreceptor TÁMOP-4.1.2-08/1/A-2009-0011 Cys-loop ion-channel receptors N Pore C C C N N N N C C TM TM TM TM 1 2 3 4 Receptor type GABAA GABAC Glycine g a b p2 p1 b a Subunit diversity a1-6, b1-3, g1-3, d,e,k, and q p1-3 a1-4, b TÁMOP-4.1.2-08/1/A-2009-0011 Vertebrate anionic Cys-loop receptors Type Class Protein name Gene Previous names a1 GABRA1 a2 GABRA2 a3 GABRA3 EJM, ECA4 alpha a4 GABRA4 a5 GABRA5 a6 GABRA6 b1 GABRB1 beta b2 GABRB2 ECA5 b3 GABRB3 g1 GABRG1 GABAA gamma g2 GABRG2 CAE2, ECA2, GEFSP3 g3 GABRG3 delta d GABRD epsilon e GABRE pi p GABRP