The Flatworm Macrostomum Lignano Is a Powerful Model Organism for Ion Channel and Stem Cell Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Temperature on the Development, Reproduction and Regeneration in the Flatworm Model Organism Macrostomum Lignano
bioRxiv preprint doi: https://doi.org/10.1101/389478; this version posted August 10, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Influence of temperature on the development, reproduction and regeneration in the flatworm model organism Macrostomum lignano Jakub Wudarski1, Kirill Ustyantsev2, Lisa Glazenburg1, Eugene Berezikov1* 1 European Research Institute for the Biology of Ageing, University of Groningen, University Medical Center Groningen, Antonius Deusinglaan 1, 9713AV, Groningen, The Netherlands; 2 Institute of Cytology and Genetics, Prospekt Lavrentyeva 10, 630090, Novosibirsk, Russia. * Correspondence: [email protected] Abstract The free-living marine flatworm Macrostomum lignano is a powerful model organism to study mechanisms of regeneration and stem cell regulation due to its convenient combination of biological and experimental properties, including the availability of transgenesis methods, which is unique among flatworm models. However, due to its relatively recent introduction in research, there are still many biological aspects of the animal that are not known. One of such questions is the influence of the culturing temperature on Macrostomum biology. Here we systematically investigated how different culturing temperatures affect the development time, reproduction rate, regeneration, heat shock response, and gene knockdown efficiency by RNA interference in M. lignano. We used marker transgenic lines of the flatworm to accurately measure the regeneration endpoint and to establish the stress response threshold for temperature shock. We found that compared to the culturing temperature of 20oC commonly used for M. -

Supplementary Material
Supplementary Material Table S1: Significant downregulated KEGGs pathways identified by DAVID following exposure to five cinnamon- based phenylpropanoids (p < 0.05). p-value Term: Genes (Benjamini) Cytokine-cytokine receptor interaction: FASLG, TNFSF14, CXCL11, IL11, FLT3LG, CCL3L1, CCL3L3, CXCR6, XCR1, 2.43 × 105 RTEL1, CSF2RA, TNFRSF17, TNFRSF14, CCNL2, VEGFB, AMH, TNFRSF10B, INHBE, IFNB1, CCR3, VEGFA, CCR2, IL12A, CCL1, CCL3, CXCL5, TNFRSF25, CCR1, CSF1, CX3CL1, CCL7, CCL24, TNFRSF1B, IL12RB1, CCL21, FIGF, EPO, IL4, IL18R1, FLT1, TGFBR1, EDA2R, HGF, TNFSF8, KDR, LEP, GH2, CCL13, EPOR, XCL1, IFNA16, XCL2 Neuroactive ligand-receptor interaction: OPRM1, THRA, GRIK1, DRD2, GRIK2, TACR2, TACR1, GABRB1, LPAR4, 9.68 × 105 GRIK5, FPR1, PRSS1, GNRHR, FPR2, EDNRA, AGTR2, LTB4R, PRSS2, CNR1, S1PR4, CALCRL, TAAR5, GABRE, PTGER1, GABRG3, C5AR1, PTGER3, PTGER4, GABRA6, GABRA5, GRM1, PLG, LEP, CRHR1, GH2, GRM3, SSTR2, Chlorogenic acid Chlorogenic CHRM3, GRIA1, MC2R, P2RX2, TBXA2R, GHSR, HTR2C, TSHR, LHB, GLP1R, OPRD1 Hematopoietic cell lineage: IL4, CR1, CD8B, CSF1, FCER2, GYPA, ITGA2, IL11, GP9, FLT3LG, CD38, CD19, DNTT, 9.29 × 104 GP1BB, CD22, EPOR, CSF2RA, CD14, THPO, EPO, HLA-DRA, ITGA2B Cytokine-cytokine receptor interaction: IL6ST, IL21R, IL19, TNFSF15, CXCR3, IL15, CXCL11, TGFB1, IL11, FLT3LG, CXCL10, CCR10, XCR1, RTEL1, CSF2RA, IL21, CCNL2, VEGFB, CCR8, AMH, TNFRSF10C, IFNB1, PDGFRA, EDA, CXCL5, TNFRSF25, CSF1, IFNW1, CNTFR, CX3CL1, CCL5, TNFRSF4, CCL4, CCL27, CCL24, CCL25, CCL23, IFNA6, IFNA5, FIGF, EPO, AMHR2, IL2RA, FLT4, TGFBR2, EDA2R, -

Research Article Microarray-Based Comparisons of Ion Channel Expression Patterns: Human Keratinocytes to Reprogrammed Hipscs To
Hindawi Publishing Corporation Stem Cells International Volume 2013, Article ID 784629, 25 pages http://dx.doi.org/10.1155/2013/784629 Research Article Microarray-Based Comparisons of Ion Channel Expression Patterns: Human Keratinocytes to Reprogrammed hiPSCs to Differentiated Neuronal and Cardiac Progeny Leonhard Linta,1 Marianne Stockmann,1 Qiong Lin,2 André Lechel,3 Christian Proepper,1 Tobias M. Boeckers,1 Alexander Kleger,3 and Stefan Liebau1 1 InstituteforAnatomyCellBiology,UlmUniversity,Albert-EinsteinAllee11,89081Ulm,Germany 2 Institute for Biomedical Engineering, Department of Cell Biology, RWTH Aachen, Pauwelstrasse 30, 52074 Aachen, Germany 3 Department of Internal Medicine I, Ulm University, Albert-Einstein Allee 11, 89081 Ulm, Germany Correspondence should be addressed to Alexander Kleger; [email protected] and Stefan Liebau; [email protected] Received 31 January 2013; Accepted 6 March 2013 Academic Editor: Michael Levin Copyright © 2013 Leonhard Linta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Ion channels are involved in a large variety of cellular processes including stem cell differentiation. Numerous families of ion channels are present in the organism which can be distinguished by means of, for example, ion selectivity, gating mechanism, composition, or cell biological function. To characterize the distinct expression of this group of ion channels we have compared the mRNA expression levels of ion channel genes between human keratinocyte-derived induced pluripotent stem cells (hiPSCs) and their somatic cell source, keratinocytes from plucked human hair. This comparison revealed that 26% of the analyzed probes showed an upregulation of ion channels in hiPSCs while just 6% were downregulated. -

Identification of Key Genes and Pathways Involved in Response To
Deng et al. Biol Res (2018) 51:25 https://doi.org/10.1186/s40659-018-0174-7 Biological Research RESEARCH ARTICLE Open Access Identifcation of key genes and pathways involved in response to pain in goat and sheep by transcriptome sequencing Xiuling Deng1,2†, Dong Wang3†, Shenyuan Wang1, Haisheng Wang2 and Huanmin Zhou1* Abstract Purpose: This aim of this study was to investigate the key genes and pathways involved in the response to pain in goat and sheep by transcriptome sequencing. Methods: Chronic pain was induced with the injection of the complete Freund’s adjuvant (CFA) in sheep and goats. The animals were divided into four groups: CFA-treated sheep, control sheep, CFA-treated goat, and control goat groups (n 3 in each group). The dorsal root ganglions of these animals were isolated and used for the construction of a cDNA= library and transcriptome sequencing. Diferentially expressed genes (DEGs) were identifed in CFA-induced sheep and goats and gene ontology (GO) enrichment analysis was performed. Results: In total, 1748 and 2441 DEGs were identifed in CFA-treated goat and sheep, respectively. The DEGs identi- fed in CFA-treated goats, such as C-C motif chemokine ligand 27 (CCL27), glutamate receptor 2 (GRIA2), and sodium voltage-gated channel alpha subunit 3 (SCN3A), were mainly enriched in GO functions associated with N-methyl- D-aspartate (NMDA) receptor, infammatory response, and immune response. The DEGs identifed in CFA-treated sheep, such as gamma-aminobutyric acid (GABA)-related DEGs (gamma-aminobutyric acid type A receptor gamma 3 subunit [GABRG3], GABRB2, and GABRB1), SCN9A, and transient receptor potential cation channel subfamily V member 1 (TRPV1), were mainly enriched in GO functions related to neuroactive ligand-receptor interaction, NMDA receptor, and defense response. -

The Free-Living Flatworm Macrostomum Lignano
ARTICLE IN PRESS Experimental Gerontology xxx (2009) xxx–xxx Contents lists available at ScienceDirect Experimental Gerontology journal homepage: www.elsevier.com/locate/expgero Review The free-living flatworm Macrostomum lignano: A new model organism for ageing research Stijn Mouton a,*, Maxime Willems a, Bart P. Braeckman b, Bernhard Egger c, Peter Ladurner c, Lukas Schärer d, Gaetan Borgonie a a Nematology Unit, Department of Biology, Ghent University, Ledeganckstraat 35, 9000 Ghent, Belgium b Laboratory for Ageing Physiology and Molecular Evolution, Department of Biology, Ghent University, Ledeganckstraat 35, 9000 Ghent, Belgium c Ultrastructural Research and Evolutionary Biology, Institute of Zoology, University of Innsbruck, Technikerstrasse 25, 6020 Innsbruck, Austria d Evolutionary Biology, Zoological Institute, University of Basel, Vesalgasse 1, 4051 Basel, Switzerland article info abstract Article history: To study the several elements and causes of ageing, diverse model organisms and methodologies are Received 5 September 2008 required. The most frequently used models are Saccharomyces cerevisiae, Caenorhabditis elegans, Drosoph- Received in revised form 6 November 2008 ila melanogaster and rodents. All have their advantages and disadvantages and allow studying particular Accepted 28 November 2008 aspects of the ageing process. During the last few years, several ageing studies focussed on stem cells and Available online xxxx their role in tissue homeostasis. Here we present a new model organism which can study this relation where other model systems fail. The flatworm Macrostomum lignano possesses a dynamic population Keywords: of likely totipotent somatic stem cells known as neoblasts. Several characteristics qualify M. lignano as Flatworm a suitable model system for ageing studies in general and more specifically for gaining more insight in Macrostomum lignano Ageing the causal relation between stem cells, ageing and rejuvenation. -

Macrostomum Lignano Jakub Wudarski1, Kirill Ustyantsev2, Lisa Glazenburg1 and Eugene Berezikov1*
Wudarski et al. Zoological Letters (2019) 5:7 https://doi.org/10.1186/s40851-019-0122-6 RESEARCH ARTICLE Open Access Influence of temperature on development, reproduction and regeneration in the flatworm model organism, Macrostomum lignano Jakub Wudarski1, Kirill Ustyantsev2, Lisa Glazenburg1 and Eugene Berezikov1* Abstract Background: The free-living marine flatworm Macrostomum lignano is a powerful model organism for use in studying mechanisms of regeneration and stem cell regulation due to its combination of biological and experimental properties, including the availability of transgenesis methods, which is unique among flatworm models. However, due to its relatively recent introduction in research, many aspects of this animal’s biology remain unknown. One such question is the influence of culture temperature on Macrostomum biology. Results: We systematically investigated how different culture temperatures affect development time, reproduction rate, regeneration, heat shock response, and gene knockdown efficiency by RNA interference (RNAi) in M. lignano. We used marker transgenic lines to accurately measure the regeneration endpoint, and to establish the stress response threshold for temperature shock. We found that compared to the culture temperature of 20 °C commonly used for M. lignano, temperatures of 25 °C–30 °C substantially increase the speed of development and regeneration, lead to faster manifestation of RNAi phenotypes, and increase reproduction rate without detectable negative consequences for the animal, while temperatures above 30 °C elicit a heat shock response. Conclusions: We show that altering temperature conditions can be used to reduce the time required to establish M. lignano cultures, perform RNAi experiments, store important lines, and optimize microinjection procedures for transgenesis. -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -
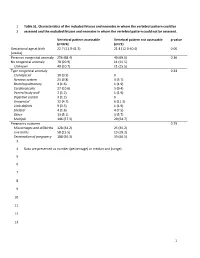
1 Table S1. Characteristics of the Included Fetuses and Neonates in Whom the Vertebral Pattern Could Be 1 Assessed and the Exclu
1 Table S1. Characteristics of the included fetuses and neonates in whom the vertebral pattern could be 2 assessed and the excluded fetuses and neonates in whom the vertebral pattern could not be assessed. Vertebral pattern assessable Vertebral pattern not assessable p-value (n=374) (n=71) Gestational age at birth 22.7 (11.9-41.3) 21.4 (12.0-40.4) 0.06 (weeks) Presence congenital anomaly 256 (68.4) 49 (69.0) 0.36 No congenital anomaly 78 (20.9) 11 (15.5) Unknown 40 (10.7) 11 (15.5) Type congenital anomaly 0.24 Craniofacial 10 (3.9) 0 Nervous system 25 (9.8) 3 (5.7) Bronchopulmonary 4 (1.6) 1 (1.9) Cardiovascular 27 (10.6) 5 (9.4) Ventral body wall 3 (1.2) 1 (1.9) Digestive system 3 (1.2) 0 Urogenital 12 (4.7) 6 (11.3) Limb defects 9 (3.5) 1 (1.9) Skeletal 4 (1.6) 4 (7.5) Other 13 (5.1) 3 (5.7) Multiple 146 (57.3) 29 (54.7) Pregnancy outcome 0.79 Miscarriages and stillbirths 128 (34.2) 25 (35.2) Live births 58 (15.5) 13 (18.3) Termination of pregnancy 188 (50.3) 33 (46.5) 3 4 Data are presented as number (percentage) or median and (range). 5 6 7 8 9 10 11 12 13 1 14 Table S2. Gestational age at birth, age at death, presence of congenital abnormalities and cause of death of the included live births. GA at birth Age at death Cervical Congenital abnormalities Cause of death (weeks) (days) rib(s) 22.3 0 No. -

Replicated Risk Nicotinic Cholinergic Receptor Genes for Nicotine Dependence
G C A T T A C G G C A T genes Article Replicated Risk Nicotinic Cholinergic Receptor Genes for Nicotine Dependence Lingjun Zuo 1, Rolando Garcia-Milian 2, Xiaoyun Guo 1,3,4,*, Chunlong Zhong 5,*, Yunlong Tan 6, Zhiren Wang 6, Jijun Wang 3, Xiaoping Wang 7, Longli Kang 8, Lu Lu 9,10, Xiangning Chen 11,12, Chiang-Shan R. Li 1 and Xingguang Luo 1,6,* 1 Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06510, USA; [email protected] (L.Z.); [email protected] (C.-S.R.L.) 2 Curriculum & Research Support Department, Cushing/Whitney Medical Library, Yale University School of Medicine, New Haven, CT 06510, USA; [email protected] 3 Shanghai Mental Health Center, Shanghai 200030, China; [email protected] 4 Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT 06510, USA 5 Department of Neurosurgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China 6 Biological Psychiatry Research Center, Beijing Huilongguan Hospital, Beijing 100096, China; [email protected] (Y.T.); [email protected] (Z.W.) 7 Department of Neurology, Shanghai First People’s Hospital, Shanghai Jiao Tong University, Shanghai 200080, China; [email protected] 8 Key Laboratory for Molecular Genetic Mechanisms and Intervention Research on High Altitude Diseases of Tibet Autonomous Region, Xizang Minzu University School of Medicine, Xianyang, Shanxi 712082, China; [email protected] 9 Provincial Key Laboratory for Inflammation and Molecular Drug Target, Medical -

The Regenerative Flatworm Macrostomum Lignano, a Model
Int. J. Dev. Biol. 62: 551-558 (2018) https://doi.org/10.1387/ijdb.180077eb www.intjdevbiol.com The regenerative flatwormMacrostomum lignano, a model organism with high experimental potential STIJN MOUTON#, JAKUB WUDARSKI#, MAGDA GRUDNIEWSKA and EUGENE BEREZIKOV* European Research Institute for the Biology of Ageing, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands ABSTRACT Understanding the process of regeneration has been one of the longstanding sci- entific aims, from a fundamental biological perspective, as well as within the applied context of regenerative medicine. Because regeneration competence varies greatly between organisms, it is essential to investigate different experimental animals. The free-living marine flatworm Macros- tomum lignano is a rising model organism for this type of research, and its power stems from a unique set of biological properties combined with amenability to experimental manipulation. The biological properties of interest include production of single-cell fertilized eggs, a transparent body, small size, short generation time, ease of culture, the presence of a pluripotent stem cell popula- tion, and a large regeneration competence. These features sparked the development of molecular tools and resources for this animal, including high-quality genome and transcriptome assemblies, gene knockdown, in situ hybridization, and transgenesis. Importantly, M. lignano is currently the only flatworm species for which transgenesis methods are established. This review summarizes -

Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology
brain sciences Review Nicotinic Receptors in Sleep-Related Hypermotor Epilepsy: Pathophysiology and Pharmacology Andrea Becchetti 1,* , Laura Clara Grandi 1 , Giulia Colombo 1 , Simone Meneghini 1 and Alida Amadeo 2 1 Department of Biotechnology and Biosciences, University of Milano-Bicocca, 20126 Milano, Italy; [email protected] (L.C.G.); [email protected] (G.C.); [email protected] (S.M.) 2 Department of Biosciences, University of Milano, 20133 Milano, Italy; [email protected] * Correspondence: [email protected] Received: 13 October 2020; Accepted: 21 November 2020; Published: 25 November 2020 Abstract: Sleep-related hypermotor epilepsy (SHE) is characterized by hyperkinetic focal seizures, mainly arising in the neocortex during non-rapid eye movements (NREM) sleep. The familial form is autosomal dominant SHE (ADSHE), which can be caused by mutations in genes encoding subunits of the neuronal nicotinic acetylcholine receptor (nAChR), Na+-gated K+ channels, as well as non-channel signaling proteins, such as components of the gap activity toward rags 1 (GATOR1) macromolecular complex. The causative genes may have different roles in developing and mature brains. Under this respect, nicotinic receptors are paradigmatic, as different pathophysiological roles are exerted by distinct nAChR subunits in adult and developing brains. The widest evidence concerns α4 and β2 subunits. These participate in heteromeric nAChRs that are major modulators of excitability in mature neocortical circuits as well as regulate postnatal synaptogenesis. However, growing evidence implicates mutant α2 subunits in ADSHE, which poses interpretive difficulties as very little is known about the function of α2-containing (α2*) nAChRs in the human brain. -

Potential Role of Genomic Imprinted Genes and Brain Developmental
Li et al. BMC Medical Genomics (2020) 13:54 https://doi.org/10.1186/s12920-020-0693-2 RESEARCH ARTICLE Open Access Potential role of genomic imprinted genes and brain developmental related genes in autism Jian Li1*† , Xue Lin2†, Mingya Wang1†,YunyunHu1, Kaiyu Xue1,ShuanglinGu1,LiLv1, Saijun Huang3 and Wei Xie1* Abstract Background: Autism is a complex disease involving both environmental and genetic factors. Recent efforts have implicated the correlation of genomic imprinting and brain development in autism, however the pathogenesis of autism is not completely clear. Here, we used bioinformatic tools to provide a comprehensive analysis of the autism-related genes, genomic imprinted genes and the spatially and temporally differentially expressed genes of human brain, aiming to explore the relationship between autism, brain development and genomic imprinting. Methods: This study analyzed the distribution correlation between autism-related genes and imprinted genes on chromosomes using sliding windows and statistical methods. The normal brains’ gene expression microarray data were reanalyzed to construct a spatio-temporal coordinate system of gene expression during brain development. Finally, we intersected the autism-related genes, imprinted genes and brain spatio-temporally differentially expressed genes for further analysis to find the major biological processes that these genes involved. Results: We found a positive correlation between the autism-related genes’ and imprinted genes’ distribution on chromosomes. Through the analysis of the normal brain microarray data, we constructed a spatio-temporal coordinate system of gene expression during human brain development, and obtained 13 genes that are differentially expressed in the process of brain development, which are both autism-related genes and imprinted genes.