Review of the Distribution and Conservation Status of the Terrestrial Reptiles of the Cape Verde Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United Nations Educational, Scientific and Cultural Organization
SC-20/CONF.232/8 Paris, 27 September 2020 Original: English UNITED NATIONS EDUCATIONAL, SCIENTIFIC AND CULTURAL ORGANIZATION International Co-ordinating Council of the Man and the Biosphere (MAB) Programme Thirty-second session Online session 27– 28 September 2020 ITEM 9 OF THE PROVISIONAL AGENDA: Proposals for New Biosphere Reserves and Extensions/ Modifications/ Renaming to Biosphere Reserves that are part of the World Network of Biosphere Reserves (WNBR) The Secretariat of the United Nations Educational Scientific and Cultural Organization (UNESCO) does not represent or endorse the accuracy or reliability of any advice, opinion, statement or other information or documentation provided by States to the Secretariat of UNESCO. The publication of any such advice, opinion, statement or other information or documentation on UNESCO’s website and/or on working documents also does not imply the expression of any opinion whatsoever on the part of the Secretariat of UNESCO concerning the legal status of any country, territory, city or area or of its boundaries. 1. Proposals for new biosphere reserves and extensions to biosphere reserves that are already part of the World Network of Biosphere Reserves (WNBR) were considered at the 26th meeting of the International Advisory Committee for Biosphere Reserves (IACBR), which met at UNESCO Headquarters from 17 to 20 February 2020. 2. The members of the Advisory Committee examined 30 proposals: 25 for new biosphere reserves including one transboundary site, two resubmissions and three requests for extensions/modification and/or renaming of already existing biosphere reserves. Five new counries submitted proposals: Andora, Cap verde, Comoros, Luxemburg an Trinidad and Tobago. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Arrival and Diversification of Mabuyine Skinks (Squamata: Scincidae) in the Neotropics Based on a Fossil-Calibrated Timetree
Arrival and diversification of mabuyine skinks (Squamata: Scincidae) in the Neotropics based on a fossil-calibrated timetree Anieli Guirro Pereira and Carlos G. Schrago Department of Genetics, Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil ABSTRACT Background. The evolution of South American Mabuyinae skinks holds significant biogeographic interest because its sister lineage is distributed across the African continent and adjacent islands. Moreover, at least one insular species, Trachylepis atlantica, has independently reached the New World through transoceanic dispersal. To clarify the evolutionary history of both Neotropical lineages, this study aimed to infer an updated timescale using the largest species and gene sampling dataset ever assembled for this group. By extending the analysis to the Scincidae family, we could employ fossil information to estimate mabuyinae divergence times and carried out a formal statistical biogeography analysis. To unveil macroevolutionary patterns, we also inferred diversification rates for this lineage and evaluated whether the colonization of South American continent significantly altered the mode of Mabuyinae evolution. Methods. A time-calibrated phylogeny was inferred under the Bayesian framework employing fossil information. This timetree was used to (i) evaluate the historical biogeography of mabuiyines using the statistical approach implemented in Bio- GeoBEARS; (ii) estimate macroevolutionary diversification rates of the South American Mabuyinae lineages and the patterns of evolution of selected traits, namely, the mode of reproduction, body mass and snout–vent length; (iii) test the hypothesis of differential macroevolutionary patterns in South American lineages in BAMM and GeoSSE; and Submitted 21 November 2016 (iv) re-evaluate the ancestral state of the mode of reproduction of mabuyines. -

Chioninia Fogoensis) Introduction to the Island of Madeira
The Herpetological Bulletin 152, 2020: 40-41 NATURAL HISTORY NOTE https://doi.org/10.33256/hb152.4041 First evidence of Fogo Island skink (Chioninia fogoensis) introduction to the island of Madeira DAVID J. CLEMENS* & STEVEN J. R. ALLAIN Cambridgeshire & Peterborough Amphibian and Reptile Group *Corresponding author e-mail: [email protected] he Madeiran wall lizard (Teira dugesii) is the only native Treptile species found on Madeira, although other species such as the Tenerife lizard (Gallotia galloti) and moorish gecko (Tarentola mauritanica) have been introduced to the island and become established (Arnold & Burton, 2002; Jesus et al., 2013). Madeira lacks any native snakes however the flower pot snake (Indotyphlops braminus) has been accidentally introduced to the island, likely by the movement of horticulture products from the Canary Islands (Jesus et al., 2013). There are a number of other reptile (and amphibian) species recorded from Madeira, however these likely represent single individuals that may have escaped captivity and therefore do not represent self-sustaining breeding populations (Malkmus, 2004). Madeira’s depauperate herpetofauna assemblage is probably due to its relative isolation in the Atlantic Ocean, being 700 km off the west coast of Africa and about 900 km from the Iberian Peninsula (Jesus et al., 2013). In mid-December 2019, an elderly couple returned to Britain from a holiday in Madeira to find a skink in their suitcase. Upon discovering the emaciated lizard they contacted the RSPCA (Royal Society for the Prevention of Cruelty to Animals) who rehomed the skink with an exotic pet dealer. From here the lizard found a permanent home with DJC (in mid-January 2020) and it has been in his care Figure 1. -

Introduced Amphibians and Reptiles in the Cuban Archipelago
Herpetological Conservation and Biology 10(3):985–1012. Submitted: 3 December 2014; Accepted: 14 October 2015; Published: 16 December 2015. INTRODUCED AMPHIBIANS AND REPTILES IN THE CUBAN ARCHIPELAGO 1,5 2 3 RAFAEL BORROTO-PÁEZ , ROBERTO ALONSO BOSCH , BORIS A. FABRES , AND OSMANY 4 ALVAREZ GARCÍA 1Sociedad Cubana de Zoología, Carretera de Varona km 3.5, Boyeros, La Habana, Cuba 2Museo de Historia Natural ”Felipe Poey.” Facultad de Biología, Universidad de La Habana, La Habana, Cuba 3Environmental Protection in the Caribbean (EPIC), Green Cove Springs, Florida, USA 4Centro de Investigaciones de Mejoramiento Animal de la Ganadería Tropical, MINAGRI, Cotorro, La Habana, Cuba 5Corresponding author, email: [email protected] Abstract.—The number of introductions and resulting established populations of amphibians and reptiles in Caribbean islands is alarming. Through an extensive review of information on Cuban herpetofauna, including protected area management plans, we present the first comprehensive inventory of introduced amphibians and reptiles in the Cuban archipelago. We classify species as Invasive, Established Non-invasive, Not Established, and Transported. We document the arrival of 26 species, five amphibians and 21 reptiles, in more than 35 different introduction events. Of the 26 species, we identify 11 species (42.3%), one amphibian and 10 reptiles, as established, with nine of them being invasive: Lithobates catesbeianus, Caiman crocodilus, Hemidactylus mabouia, H. angulatus, H. frenatus, Gonatodes albogularis, Sphaerodactylus argus, Gymnophthalmus underwoodi, and Indotyphlops braminus. We present the introduced range of each of the 26 species in the Cuban archipelago as well as the other Caribbean islands and document historical records, the population sources, dispersal pathways, introduction events, current status of distribution, and impacts. -

Common House Gecl~O Hemidactylus /Renatus Scljegel (Squamata: Gelj~Onidae) in Cuba, with Comments on the Other Colonizing Species of the Genus in the Island
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNAL IRCF REPTILES &HIBIANS • 21(1):30-34 • MAR2014 CONSERVATION AND NATURAL HISTORY INTRODUCED SPECIES A New Locality Record for the Common House Gecl~o Hemidactylus /renatus SclJegel (Squamata: GelJ~onidae) in Cuba, with Comments on the Other Colonizing Species of the Genus in the Island Luis M. Díaz Museo Nacional de Historia Natural de Cuba, Obispo #61, esquina Oficios, Plaza de Armas, Habana Vieja, CP 10100, Cuba ([email protected] and luisfi:[email protected]) Photographs by thc author. he Common House Gecko (Hemidactylus frenatus Arnold 2006, Bomford et al. 2009). Shigesada et al. (1995) T Schlegel) is one of several species in the genus that have suggested that introduced species of Hemidactylus exhibit a colonized many places in both the Eastern and Western stratified diffusion invasion by combining human-assisted Hemispheres (i.e., Townsend and Krysko 2003, Carranza transportation and a gradual colonization of neighboring and Arnold 2006, Bomford et al. 2009, Kraus 2009, Caicedo lo calities (Meshaka 1995, Caicedo-Portilla and Dulcey-Cala Portilla and Dulcey-Cala 201 L Powell et al. 20 1 L Ota and 2011). For example, vehicles transporting household furni Whitaker 2013). Those introductions were largely medi ture and other items are known to contribute to dispersion ated by human activity and, as a consequence, the species (e.g., Somma et al. 20 13). An advantage for H frenatus in has increased its range considerably during the last century particular as a successful colonizer is the capacity for long (Rodder et al. 2008). The presence of these geckos was docu term sperm storage by females (Murphy-Walker and Haley mented recently in the Dominican Republic (Scantlebury et 1996, Yamamoto and Ota 2006). -

La Collezione Erpetologica Del Museo Civico Di Storia Naturale “G. Doria” Di Genova the Herpetological Collection of the Museo Civico Di Storia Naturale “G
MUSEOLOGIA SCIENTIFICA MEMORIE • N. 5/2010 • 62-68 Le collezioni erpetologiche dei Musei italiani The herpetological collections of italian museums Stefano Mazzotti (ed.) La collezione erpetologica del Museo Civico di Storia Naturale “G. Doria” di Genova The herpetological collection of the Museo Civico di Storia Naturale “G. Doria” of Genoa Giuliano Doria Museo Civico di Storia Naturale “G. Doria”, Via Brigata Liguria 9. I-16121 Genova. E-mail: [email protected] RIASSUNTO Il primo nucleo della collezione erpetologica del Museo Civico di Storia Naturale “Giacomo Doria” di Genova è costituito dalle raccolte effettuate da Giacomo Doria, fondatore del Museo, nella zona di La Spezia, in Persia (oggi Iran) e in Borneo (insieme a Odoardo Beccari) negli anni 1862-1868. Successivamente la collezione viene incrementata col materiale di numerose spedizioni condotte in tutti i conti - nenti; i risultati di tali raccolte sono stati spesso pubblicati sugli “Annali” del Museo. Nella collezione sono pre - senti 593 specie di Anfibi e 1.456 di Rettili; 171 taxa, attualmente validi, sono rappresentati da tipi. Parole chiave: Anfibi, Rettili, Museo di Genova, annali, tipi. ABSTRACT The first nucleus of the herpetological collection of the Museo Civico di Storia Naturale “Giacomo Doria” (Italy, Genoa) was made up of the specimens collected in the years 1862-1868 near La Spezia (Italy, Liguria), in Persia (now Iran) and in Borneo (with Odoardo Beccari) by its founder, Giacomo Doria. Later, it was increased with thousands of specimens collected during several expeditions throughout all the continents. Many important studies about this rich material have been published in “Annali”, the museum’s journal. -

An Integrative Taxonomic Revision of the Cape Verdean Skinks (Squamata, Scincidae)
Zoologica Scripta An integrative taxonomic revision of the Cape Verdean skinks (Squamata, Scincidae) AURE´ LIEN MIRALLES*, RAQUEL VASCONCELOS*, ANA PERERA,DAVID J. HARRIS &SALVADOR CARRANZA Submitted: 12 March 2010 Miralles, A., Vasconcelos, R., Perera, A., Harris, D. J. & Carranza, S. (2010). An integra- Accepted: 15 September 2010 tive taxonomic revision of the Cape Verdean skinks (Squamata, Scincidae). — Zoologica doi:10.1111/j.1463-6409.2010.00453.x Scripta, 00, 000–000. A comprehensive taxonomic revision of the Cape Verdean skinks is proposed based on an integrative approach combining (i) a phylogenetic study pooling all the previously pub- lished molecular data, (ii) new population genetic analyses using mitochondrial and nuclear data resulting from additional sampling, together with (iii) a morphological study based on an extensive examination of the scalation and colour patterns of 516 live and museum spec- imens, including most of the types. All Cape Verdean species of skinks presently recogni- sed, formerly regarded as members of the genera Mabuya Fitzinger, 1826 and Macroscincus Bocage, 1873 are considered as members of the Cape Verdean endemic genus Chioninia Gray, 1845. The new phylogeny and networks obtained are congruent with the previously published phylogenetic studies, although suggesting older colonization events (between 11.6 and 0.8 Myr old), and indicate the need for taxonomic changes. Intraspecific diversity has been analysed and points to a very recent expansion of Chioninia delalandii on the southern islands and its introduction on Maio, to a close connection between Chioninia stangeri island populations due to Pleistocene sea-level falls and to a generally low haplo- typic diversity due to the ecological and geological characteristics of the archipelago. -

Systematics, Biogeography, and Evolution of Hemidactylus Geckos (Reptilia: Gekkonidae) Elucidated Using Mitochondrial DNA Sequences
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 38 (2006) 531–545 www.elsevier.com/locate/ympev Systematics, biogeography, and evolution of Hemidactylus geckos (Reptilia: Gekkonidae) elucidated using mitochondrial DNA sequences S. Carranza a,*, E.N. Arnold b a Departament de Biologia Animal, Universitat de Barcelona, Av. Diagonal 645, E-08028 Barcelona, Spain b Department of Zoology, The Natural History Museum, London SW7 5BD, UK Received 11 May 2005; revised 12 July 2005 Available online 9 September 2005 Abstract With more than 80 species inhabiting all warm continental land masses and hundreds of intervening continental and oceanic islands, Hemidactylus geckos are one of the most species-rich and widely distributed of all reptile genera. They consequently rep- resent an excellent model for biogeographic, ecological, and evolutionary studies. A molecular phylogeny for Hemidactylus is pre- sented here, based on 702 bp of mtDNA (303 bp cytochrome b and 399 bp 12S rRNA) from 166 individuals of 30 species of Hemidactylus plus Briba brasiliana, Cosymbotus platyurus, and several outgroups. The phylogeny indicates that Hemidactylus may have initially undergone rapid radiation, and long-distance dispersal is more extensive than in any other reptilian genus. In the last 15 My, African lineages have naturally crossed the Atlantic Ocean at least twice. They also colonized the Gulf of Guinea, Cape Verde and Socotra islands, again sometimes on more than one occasion. Many extensive range extensions have occurred much more recently, sometimes with devastating consequences for other geckos. These colonizations are likely to be largely anthropogen- ic, involving the ÔweedyÕ commensal species, H. brookii s. lat, H. -
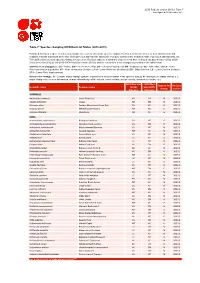
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Ríos-Franceschi Et Al. 2016
Life: The Excitement of Biology 3(4) 254 Spatiotemporal Changes of the Herpetofaunal Community in Mount Resaca and Luis Peña Cay, Culebra National Wildlife Refuge, Culebra, Puerto Rico1 Alejandro Ríos-Franceschi2,3, Juan G. García-Cancel3, Fernando J. Bird-Picó3, and Luis D. Carrasquillo3 Abstract: Culebra, which is an archipelago that forms part of the Puerto Rican Bank, has had a limited scope of biological studies, provided the basis for this work. Culebra’s terrestrial resources were disturbed since the early 1900’s until the 1970’s. Since the 1970’s, a natural reserve, called the Culebra National Wildlife Refuge, has been managed by the United States Fish and Wildlife Service. The purpose of this research is to update the species list of reptiles and amphibians on the Island as well as to examine how spatial and temporal changes affect the diversity and abundance of its herpetofauna. Twenty species of reptiles and amphibians placed in thirteen families were identified. Two new records for Mount Resaca are Eleutherodactylus coqui Thomas, 1966 and Eleutherodactylus cochranae Grant, 1932. Meanwhile, Anolis pulchellus Duméril and Bibron, 1837 is a new record for the Luis Peña Cay. Mount Resaca has greater species richness, ten more species than the Luis Peña Cay, three species of amphibians and seven species of reptiles. The differences in herpetofaunal biodiversity (e.g. Shannon Wiener, Simpson’s Index and Margalef’s Index) between Mount Resaca and the Luis Peña Cay were statistically significant. Abiotic factors, such as temperature and humidity, and biotic factors, such as vegetation and the presence of other animal species, possibly influence the relative abundances within these communities. -

A Peek Into the Evolutionary Origin of Skinks of the Indian Subcontinent
A peek into the evolutionary origin of skinks of the Indian subcontinent Aniruddha Datta-Roy CES, IISc. SCINCIDAE Scincinae Lygosominae Acontinae Indian SE Asian Eurasian African endemics Dasia Ablepharus Trachylepis Barkudia Eutropis Asymblepharus Chalcides (?) Kaestlea (Mabuya) Eurylepis Ristella Lygosoma Ophiomorus Sepsophis Sphenomorphus Tropidophorus Systematics and Biogeography of Eutropis (Squamata: Scincidae) from the Indian subcontinent Datta-Roy et. al.2012 Genus Mabuya • Subfamily Lygosominae • Circumtropical distribution • Until recently one of the most speciose genera • Divided into – Eutropis, Chioninia, Mabuya, Trachylepis Trachylepis Mabuya Chioninia Eutropis Mausfeld et. al., 2000, 2002 S. No Genus and species Global Distribution 1 Mabuya macularia Indian subregion, SE Asia 2 Mabuya carinata Endemic, Indian subregion 3 Mabuya dissimilis Indian subregion, SE Asia 4 Mabuya allapallensis Endemic, Peninsular India 5 Mabuya beddomii Endemic, Peninsular India 6 Mabuya bibronii Endemic, Peninsular India 7 Mabuya clivicola Endemic, Peninsular India 8 Mabuya floweri Endemic, Peninsular India 9 Mabuya gansi Endemic, Peninsular India 10 Mabuya innotata Endemic, Peninsular India 11 Mabuya madaraszi Endemic, Peninsular India 12 Mabuya nagarjuni Endemic, Peninsular India 13 Mabuya tamanna Endemic, Peninsular India 14 Mabuya trivittata Endemic, Peninsular India Smith, 1935, Das, 1998 2003, Reptile Database.org, S. No Genus and species Global Distribution 1 Eutropis macularia Indian subregion, SE Asia 2 Eutropis carinata Endemic, Indian