Species Which Remained Immutable and Unchanged Thereafter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Stars: Introduction
Neutron Stars: Introduction Stephen Eikenberry 16 Jan 2018 Original Ideas - I • May 1932: James Chadwick discovers the neutron • People knew that n u clei w ere not protons only (nuclear mass >> mass of protons) • Rutherford coined the name “neutron” to describe them (thought to be p+ e- pairs) • Chadwick identifies discrete particle and shows mass is greater than p+ (by 0.1%) • Heisenberg shows that neutrons are not p+ e- pairs Original Ideas - II • Lev Landau supposedly suggested the existence of neutron stars the night he heard of neutrons • Yakovlev et al (2012) show that he in fact discussed dense stars siiltimilar to a gi ant nucl eus BEFORE neutron discovery; this was immediately adapted to “t“neutron s t”tars” Original Ideas - III • 1934: Walter Baade and Fritz Zwicky suggest that supernova eventtttts may create neutron stars • Why? • Suppg(ernovae have huge (but measured) energies • If NS form from normal stars, then the gravitational binding energy gets released • ESN ~ star … Original Ideas - IV • Late 1930s: Oppenheimer & Volkoff develop first theoretical models and calculations of neutron star structure • WldhWould have con tidbttinued, but WWII intervened (and Oppenheimer was busy with other things ) • And that is how things stood for about 30 years … Little Green Men - I • Cambridge experiment with dipole antennae to map cosmic radio sources • PI: Anthony Hewish; grad students included Jocelyn Bell Little Green Men - II • August 1967: CP 1919 discovered in the radio survey • Nov ember 1967: Bell notices pulsations at P =1.337s -

Beeps, Flashes, Bangs and Bursts
Beeps, Flashes, Big stars work much faster: Bangs and Bursts. 1,000,000 100,000 100,000 100,000 and Chirps. Forever Peter Watson 3 hours! Live fast, Die Young! Peter Watson, Dept. of Physics Change colour, size, brightness •Vast majority of stars are boring: “main- sequence” (aka middle- class) changing very slowly. •Some oscillate: e.g Cepheids •Large bright stars change by factor 3 in brightness Peter Watson Peter Watson If Stars are large.... • we get supernovae • 6 visible in Milky Way over last 1000 years •well understood: work by blocking mechanism • SN 1006: Brightest •very important since period is proportional to Supernova. intrinsic brightness: • Can see remnants of the expanding •i.e. measure the apparent brightness, the period tells shockwave you the actual brightness, so you know how far away Frank Winkler (Middlebury College) et it is al., AURA, NOAO, NSF Peter Watson Peter Watson Remnant of a very Tycho’s old SN Supernova • Part of the veil nebula in X-rays in Cygnus (1572) Sara Wager NASA / CXC / F.J. Lu (Chinese Academy of Sciences) et al. Peter Watson Peter Watson The Crab (M1) •Recorded by Chinese astronomers "I humbly observe that a guest star has appeared; above the star there is a feeble yellow glimmer. If one examines the divination regarding the Emperor, the interpretation [of the presence of this guest star] is the following: The fact that the star has not overrun Bi and that its brightness must represent a person of great value. I demand that the Office of Historiography is informed of this." PW Peter Watson 1054: Crab •Recorded by Chinese astronomers as “guest star” •May have been recorded by Chaco Indians in New • X-rays (in blue) Mexico • + Optical 4 a.m. -

The Korean 1592--1593 Record of a Guest Star: Animpostor'of The
Journal of the Korean Astronomical Society 49: 00 ∼ 00, 2016 December c 2016. The Korean Astronomical Society. All rights reserved. http://jkas.kas.org THE KOREAN 1592–1593 RECORD OF A GUEST STAR: AN ‘IMPOSTOR’ OF THE CASSIOPEIA ASUPERNOVA? Changbom Park1, Sung-Chul Yoon2, and Bon-Chul Koo2,3 1Korea Institute for Advanced Study, 85 Hoegi-ro, Dongdaemun-gu, Seoul 02455, Korea; [email protected] 2Department of Physics and Astronomy, Seoul National University, Gwanak-gu, Seoul 08826, Korea [email protected], [email protected] 3Visiting Professor, Korea Institute for Advanced Study, Dongdaemun-gu, Seoul 02455, Korea Received |; accepted | Abstract: The missing historical record of the Cassiopeia A (Cas A) supernova (SN) event implies a large extinction to the SN, possibly greater than the interstellar extinction to the current SN remnant. Here we investigate the possibility that the guest star that appeared near Cas A in 1592{1593 in Korean history books could have been an `impostor' of the Cas A SN, i.e., a luminous transient that appeared to be a SN but did not destroy the progenitor star, with strong mass loss to have provided extra circumstellar extinction. We first review the Korean records and show that a spatial coincidence between the guest star and Cas A cannot be ruled out, as opposed to previous studies. Based on modern astrophysical findings on core-collapse SN, we argue that Cas A could have had an impostor and derive its anticipated properties. It turned out that the Cas A SN impostor must have been bright (MV = −14:7 ± 2:2 mag) and an amount of dust with visual extinction of ≥ 2:8 ± 2:2 mag should have formed in the ejected envelope and/or in a strong wind afterwards. -

G7. 7-3.7: a Young Supernova Remnant Probably Associated with the Guest
Draft version September 12, 2018 Typeset using LATEX twocolumn style in AASTeX62 G7.7-3.7: a young supernova remnant probably associated with the guest star in 386 CE (SN 386) Ping Zhou (hs),1, 2 Jacco Vink,1, 3, 4 Geng Li (Î耕),5, 6 and Vladim´ır Domcekˇ 1, 3 1Anton Pannekoek Institute for Astronomy, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands 2School of Astronomy and Space Science, Nanjing University, 163 Xianlin Avenue, Nanjing, 210023, China 3GRAPPA, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands 4SRON, Netherlands Institute for Space Research, Sorbonnelaan 2, 3584 CA Utrecht, The Netherlands 5National Astronomical Observatories, Chinese Academy of Sciences, 20A Datun Road, Chaoyang District, Beijing 100101, China 6School of Astronomy and Space Science, University of Chinese Academy of Sciences, No.19A Yuquan Road, Shijingshan District, Beijing 100049, China Submitted to ApJL ABSTRACT Although the Galactic supernova rate is about 2 per century, only few supernova remnants are associated with historical records. There are a few ancient Chinese records of \guest stars" that are probably sightings of supernovae for which the associated supernova remnant is not established. Here we present an X-ray study of the supernova remnant G7.7−3:7, as observed by XMM-Newton, and discuss its probable association with the guest star of 386 CE. This guest star occurred in the ancient Chinese asterism Nan-Dou, which is part of Sagittarius. The X-ray morphology of G7.7−3:7 shows an arc-like feature in the SNR south, which is characterized by an under-ionized plasma with sub-solar abundances, a temperature of 0:4{0.8 keV, and a density of ∼ 0:5(d=4 kpc)−0:5 cm−3. -

Neutron Stars and Their Envelopes A.Y.Potekhin1,2 in Collaboration with G.Chabrier,2 A.D.Kaminker,1 D.G.Yakovlev,1
Dense matter in neutron stars and their envelopes A.Y.Potekhin1,2 in collaboration with G.Chabrier,2 A.D.Kaminker,1 D.G.Yakovlev,1 ... 1Ioffe Physical-Technical Institute, St.Petersburg 2CRAL, Ecole Normale Supérieure de Lyon Introduction: Neutron stars and their importance for fundamental physics Neutron-star envelopes – link between the superdense core and observations Conductivities and thermal structure of neutron star envelopes Atmospheres and thermal radiation spectra of neutron stars with magnetic fields Neutron stars – the densest stars in the Universe Mass and radius: Gravitational energy: average density: Neutron stars on the density – temperature diagram Phase diagram of dense matter. Courtesy of David Blaschke GR effects Gravitational radius Redshift zg: “compactness parameter” u=rg/R ~ 0.3–0.4 “Observed” temperature = Teff /(1+zg) gravity Light rays are bending near the stellar surface, thus allowing one to “look behind the horizon”. “Apparent” radius Neutron stars – the stars with the strongest magnetic field Radio waves P ≈ 1.4 ms – 12 s Ω ≈ 0.5 – 4500 s−1 B In the strong magnetic field of a rapidly rotating neutron star, charged particles are accelerated to relativistic energies, creating coherent radio emission. Therefore many neutron stars are observed as pulsars. Ω2 B2 Ω1 ω B1 Gravitational waves Radio waves Binary neutron stars emit gravitational waves (losing the angular momentum) and undergo relativistic precession. Prediction L.D.Landau (1931) – anticipation [L.D.Landau, “On the theory of stars,” Physikalische Zs. Sowjetunion 1 (1932) 285]: for stars with M>1.5M☼ “density of matter becomes so great that atomic nuclei come in close contact, foming one gigantic nucleus’’. -

Variable Star
Variable star A variable star is a star whose brightness as seen from Earth (its apparent magnitude) fluctuates. This variation may be caused by a change in emitted light or by something partly blocking the light, so variable stars are classified as either: Intrinsic variables, whose luminosity actually changes; for example, because the star periodically swells and shrinks. Extrinsic variables, whose apparent changes in brightness are due to changes in the amount of their light that can reach Earth; for example, because the star has an orbiting companion that sometimes Trifid Nebula contains Cepheid variable stars eclipses it. Many, possibly most, stars have at least some variation in luminosity: the energy output of our Sun, for example, varies by about 0.1% over an 11-year solar cycle.[1] Contents Discovery Detecting variability Variable star observations Interpretation of observations Nomenclature Classification Intrinsic variable stars Pulsating variable stars Eruptive variable stars Cataclysmic or explosive variable stars Extrinsic variable stars Rotating variable stars Eclipsing binaries Planetary transits See also References External links Discovery An ancient Egyptian calendar of lucky and unlucky days composed some 3,200 years ago may be the oldest preserved historical document of the discovery of a variable star, the eclipsing binary Algol.[2][3][4] Of the modern astronomers, the first variable star was identified in 1638 when Johannes Holwarda noticed that Omicron Ceti (later named Mira) pulsated in a cycle taking 11 months; the star had previously been described as a nova by David Fabricius in 1596. This discovery, combined with supernovae observed in 1572 and 1604, proved that the starry sky was not eternally invariable as Aristotle and other ancient philosophers had taught. -

Chapter 13 – the Deaths of Stars
Chapter 13 – The Deaths of Stars On July 4, 1054, Chinese astronomers noted a new bright star in the constellation of Taurus. The “guest star” was so bright it could be seen in daylight for almost a month! This event was recorded by several independent chinese astronomers and could be observed for almost 2 years. This dramatic event heralded the death throes of a massive star only 6500 light years away. When we turn modern day telescopes towards Taurus we observe the fallout from this immense explosion. This region is known as the Crab Nebula (M1). Located inside the nebula is a pulsar – a fast rotating neutron star, the final dense remains of the star that went supernova only a millenium ago... As with many properties of stars that we have seen, how a star ends its life depends on its mass. The common feature across the mass range is that stars “die” when they run out of fuel, because now there is no way to resist the compression force of gravity. The Death of Low Mass Stars The core of a star heats up in response to gravitational contraction. Therefore, low mass stars never get very hot. In fact, stars below 0.4 solar masses only get hot enough to fuse hydrogen (and only in p-p chain reactions). This low temperature means that the hydrogen fuel is consumed relatively slowly. In addition, as we saw in a previous lecture, very low mass stars are completely convective. The large scale convection continually brings fresh hydrogen fuel to the centre of the star. -

Neutron Star
Phys 321: Lecture 8 Stellar Remnants Prof. Bin Chen, Tiernan Hall 101, [email protected] Evolution of a low-mass star Planetary Nebula Main SequenceEvolution and Post-Main-Sequence track of an 1 Stellar solar Evolution-mass star Post-AGB PN formation Superwind First He shell flash White dwarf TP-AGB Second dredge-up He core flash ) Pre-white dwarf L / L E-AGB ( 10 He core exhausted Log RGB Ring Nebula (M57) He core burning First Red: Nitrogen, Green: Oxygen, Blue: Helium dredge-up H shell burning SGB Core contraction H core exhausted ZAMS To white dwarf phase 1 M Log (T ) 10 e Sirius B FIGURE 4 A schematic diagram of the evolution of a low-mass star of 1 M from the zero-age main sequence to the formation of a white dwarf star. The dotted ph⊙ase of evolution represents rapid evolution following the helium core flash. The various phases of evolution are labeled as follows: Zero-Age-Main-Sequence (ZAMS), Sub-Giant Branch (SGB), Red Giant Branch (RGB), Early Asymptotic Giant Branch (E-AGB), Thermal Pulse Asymptotic Giant Branch (TP-AGB), Post- Asymptotic Giant Branch (Post-AGB), Planetary Nebula formation (PN formation), and Pre-white dwarf phase leading to white dwarf phase. and becomes nearly isothermal. At points 4 in Fig. 1, the Schönberg–Chandrasekhar limit is reached and the core begins to contract rapidly, causing the evolution to proceed on the much faster Kelvin–Helmholtz timescale. The gravitational energy released by the rapidly contracting core again causes the envelope of the star to expand and the effec- tive temperature cools, resulting in redward evolution on the H–R diagram. -
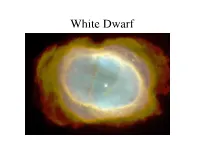
White Dwarf Properties of a White Dwarf
White Dwarf Properties of a White Dwarf • Contains most of star’s original mass • Yet diameter is roughly Earth’s diameter • Density about 3x109 kg/m3 One teaspoon would weigh 15 tons Surface gravity = 1.3 x 105 g vesc = 0.02 x speed of light • Teff = 10,000 K, MV = +11 (Sun is +4.8) White Dwarf • After planetary nebula dissipates, all that’s left is the hot degenerate core • Remnant core of M<8 Msun star • M<4Msun: C-O white dwarf (never ignited C) • M=4-8Msun: O-Ne-Mg white dwarf • Supported by electron degeneracy pressure • No fusion, no contraction, just cooling. For eternity. Properties of a White Dwarf Structure of isolated WD Degenerate CO core (5000 km) Thin (100m) atmosphere Structure of isolated WD •At these densities, WD is supported by electron degeneracy pressure. • This has a profound impact on its structure (M vs. R) Structure of isolated WD • WD consists of gas of ionized C+O nuclei and electrons (overall neutral) • Electrons are fermions, i.e. spin±1/2 particles (protons & neutrons as well) • 2 important quantum-mechanical things: Pauli exclusion principle; Heisenberg uncertainty principle Structure of isolated WD • Pauli exclusion principle: no 2 identical fermions can occuply exact same quantum mechanical state (e.g., energy, angular momentum, spin) • Heisenberg uncertainty principle: it is impossible to define position and momentum simultaneously to accuracy better than ~Planck’s constant for the product of the uncertainties: px is x-component (Δx)(Δpx) ≥ ћ/2 of momentum Structure of isolated WD Chandrasekhar mass limit There is an upper limit to the mass of a WD of 1.4 solar masses, set by electron degeneracy pressure… Above this limit, WD is unstable Upper limit on mass of white dwarfs Electron degeneracy can’t support more than 1.4 Msun Mass transfer in binary systems • Isolated white dwarf boring, simply cools forever (makes them good “clocks”) • White dwarfs in binary systems are more interesting • If binary stars are close enough (<1 AU), mass transfer can occur… Stars in a binary system may have more complex evolution…. -
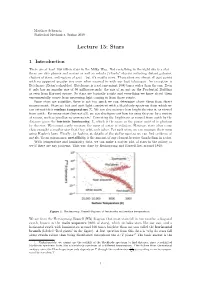
Lecture 15: Stars
Matthew Schwartz Statistical Mechanics, Spring 2019 Lecture 15: Stars 1 Introduction There are at least 100 billion stars in the Milky Way. Not everything in the night sky is a star there are also planets and moons as well as nebula (cloudy objects including distant galaxies, clusters of stars, and regions of gas) but it's mostly stars. These stars are almost all just points with no apparent angular size even when zoomed in with our best telescopes. An exception is Betelgeuse (Orion's shoulder). Betelgeuse is a red supergiant 1000 times wider than the sun. Even it only has an angular size of 50 milliarcseconds: the size of an ant on the Prudential Building as seen from Harvard square. So stars are basically points and everything we know about them experimentally comes from measuring light coming in from those points. Since stars are pointlike, there is not too much we can determine about them from direct measurement. Stars are hot and emit light consistent with a blackbody spectrum from which we can extract their surface temperature Ts. We can also measure how bright the star is, as viewed from earth . For many stars (but not all), we can also gure out how far away they are by a variety of means, such as parallax measurements.1 Correcting the brightness as viewed from earth by the distance gives the intrinsic luminosity, L, which is the same as the power emitted in photons by the star. We cannot easily measure the mass of a star in isolation. However, stars often come close enough to another star that they orbit each other. -

Rutger Van Haasteren (Jet Propulsion Lab) Detecting Gravitational-Waves
Detecting gravitational-waves by observing pulsars, nature's most accurate natural clocks. Rutger van Haasteren (Jet Propulsion Lab) Credit: David Champion Outline 1. Gravitational-wave detector principles 2. Pulsars and pulsar timing 3. Examples of pulsar timing 4. Gravitational-waves sources 5. Pulsar timing arrays 6. Outlook and detection prospects What is a GW? Gravitational wave: ripple in Gravity wave: refer to one of in the curvature of spacetime Tokyo's local surfers that propagates outward from the source as a wave. Effect and detectability of GWs Effect of GWs is an oscillating Speed of light is constant. Riemann curvature tensor, possible in two polarisations. Measure time, not distance. → Measure propagation length! Effect and detectability of GWs a b Credit: Advanced Technology Center, NAOJ Emit light, and reflect back LASER has precise frequency → equivalent to clock Now it is truly a 'timing experiment' Interferometry for detection Need precise frequency/clock Could say that KAGRA uses What about pulsar's spin a LASER as an accurate frequency? frequency standard Period of PSR B1937+21: T = 0.00155780644887275 s Strain sensitivity per frequency Energy density function of wavelength Electromagnetic waves: Ω∝∣E∣2+∣B∣2 Gravitational waves: Ω∝∣h˙∣2=f 2h(f )2 Atomic nucleus is ~1e-15m With 3km arm, reach sensitivity down to distance variations of ~1e-21m?? (zepto-meter) Yardley et al. (2010) Pulsars Discovery: LGM1 Pulsar discovery in 1967: LGM1 (= PSR B1919+21) 'Knocking sound' Discovery: LGM1 Pulsar discovery in 1967: LGM1 (= PSR B1919+21) 'Knocking sound' Explanation: neutron star Baade & Zwicky in 1934: "With all reserve we advance the view that a supernova represents the transition of an ordinary star into a new form of star, the neutron star, which would be the end point of stellar evolution. -

Nature's Biggest Explosions: Past, Present, and Future
Nature’s Biggest Explosions: Past, Present, and Future Edo Berger Harvard University Why Study Cosmic Explosions? Why Study Cosmic Explosions? Why Study Cosmic Explosions? Why Study Cosmic Explosions? Why Study Cosmic Explosions? The Past About 10 “guest stars” have been mentioned in historical records, spanning from 185 to 1604 AD. All were observed with the naked eye (first telescope was built in 1608 AD). “Throughout all past time, according to the records handed down from generation to generation, nothing is observed to have changed either in the whole of the outermost heaven or in any of its proper parts.” Aristotle, De caelo (On the Heavens), 350 BC SN 185 In 185 AD Chinese records mark the appearance of a “guest star” which remained visible for 8 months and did not move like a planet or a comet. This is the oldest record of a supernova. “In the 2nd year of the epoch Zhongping, the 10th month, on the day Kwei Hae, a strange star appeared in the middle of Nan Mun … In the 6th month of the succeeding year it disappeared.” SN 1006 On April 1006 records from Europe, the Middle East, and Asia mark the appearance of the brightest “guest star” ever seen: bright as a quarter moon and visible during the day. It remained visible for almost 2 years. “…spectacle was a large circular body, 2½ to 3 times as large as Venus. The sky was shining because of its light. The intensity of its light was a little more than the light of the Moon when one-quarter illuminated" SN 1054 On July 4, 1054 AD records from the Middle East and Asia (and potentially North America) mark the appearance of a bright “guest star”; as bright as a 1/16 moon and remained visible for 2 years.