1 1.0 Introduction 2.0 Mineralogy 3.0 Textures Exhibited by Manganese
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HIGHLIGHTS of THIS ISSUE This Listing Does Not Affect the Legal Status of Any Document Published in This Issue
WEDNESDAY, OCTOBER 27, 1971 WASHINGTON, D.C. Volume 36 ■ Number 207 Pages 20569-20640 HIGHLIGHTS OF THIS ISSUE This listing does not affect the legal status of any document published in this issue. Detailed table of contents appears inside. PEANUTS— USDA regulation proclaiming 1972 marketing quotas and acreage allotments; effec tive 10-26-71 __________ ____ _____ - 20575 COTTON LOAN PROGRAM— USDA regulations on redemptions; effective 10-26-71-------------- 20577 BURLEY TOBACCO— USDA regulation on grade rates for advances to producers on 1971 crop; effective 10-26-71................ .........— .............. 20577 AIR CARRIERS— CAB regulation on use of rec ords on microfilm and microfiche; effective 10-27-71 ................. ......... ...................... 20579 CONTRACT APPEALS— NASA regulations; effec tive 11-15-71_________ _______________________ ___ 20580 UNFAIR TRADE PRACTICES— FTC cease and desist orders on false advertising (17 docu ments) ........................... ..............................20584-20596 ANTIBIOTIC DRUGS— FDA regulation revoking certification of 8 drugs; effective within 40 days unless stayed by objections____________ _____ ____ 20597 INCOME TAX— 1RS regulations relating to examinations of incomes of churches........... ......... 20599 AVAILABILITY OF RECORDS— EEOC rule setting schedule of fees; effective 10-27-71...... ............ 20600 COAL MINE HEALTH AND SAFETY— Interior Dept, regulations for transfer of miners with evidence of pneumoconiosis.................... 20600 Interior Dept, proposal of standards -

Gamblin Provides Is the Desire to Help Painters Choose the Materials That Best Support Their Own Artistic Intentions
AUGUST 2008 Mineral and Modern Pigments: Painters' Access to Color At the heart of all of the technical information that Gamblin provides is the desire to help painters choose the materials that best support their own artistic intentions. After all, when a painting is complete, all of the intention, thought, and feeling that went into creating the work exist solely in the materials. This issue of Studio Notes looks at Gamblin's organization of their color palette and the division of mineral and modern colors. This visual division of mineral and modern colors is unique in the art material industry, and it gives painters an insight into the makeup of pigments from which these colors are derived, as well as some practical information to help painters create their own personal color palettes. So, without further ado, let's take a look at the Gamblin Artists Grade Color Chart: The Mineral side of the color chart includes those colors made from inorganic pigments from earth and metals. These include earth colors such as Burnt Sienna and Yellow Ochre, as well as those metal-based colors such as Cadmium Yellows and Reds and Cobalt Blue, Green, and Violet. The Modern side of the color chart is comprised of colors made from modern "organic" pigments, which have a molecular structure based on carbon. These include the "tongue- twisting" color names like Quinacridone, Phthalocyanine, and Dioxazine. These two groups of colors have unique mixing characteristics, so this organization helps painters choose an appropriate palette for their artistic intentions. Eras of Pigment History This organization of the Gamblin chart can be broken down a bit further by giving it some historical perspective based on the three main eras of pigment history – Classical, Impressionist, and Modern. -

What Are Ochre and Umber?
What a「e O⊂hre and Umber? 100% Natu「al I「on Oxide Pigments 〃柵e most economic。l p毎men誌fn the血dustγ’’ ochre (PrOnOunCed o′-ker) is a natura一′ mineral′ earth pigment. Chemic訓y′ it is a hyd「ated ferric oxide′ Chemical formulation: FeO(OH). Och「e is inorganic, Chemica一一y ine「t′ nOn-reaCtive with cement′ mO「tar Or brick′ and New Riverside produces two grades ofOchre pigments that are caIled 548 0chre and NIROX412. 548 Ochre is s=ghtlydarke「・ NIROX412 is mo「e yel看ow and has a greater tint strength. ochre is mined and processed in Cartersv紺e′ GAatthe oniyyear-rOund Ochre mInlng and processing operation in the United States. How応O`hre used? Ochre can be used aIone as a singie pigmentto achieve a range ofcoIors from light bu什to dark bu什simpIy byvaryingthe addition rate. As a wayto achieve a broader range ofcoIorswith a higheryeIIow or red shade, Ochre mayaIso be blended with synthetic pigments and used as a cost effective base. 548 0chre is a brown-yeIIow′ refined natu「al iron OXide pigment (Fe203) with an average particIe Size of-3 microns. it isthe mosteconomicaI bu什 COIor in the pigment industry. Broad appIication use for aii cement appiications. 5櫛くわhIe Umber is a contro看led productfrom a manganese enriched fo「m ofgoethite, a natur訓yoccurrIng InOrganic iron oxide. it is a brown earth pigmentthat is darkerthan the Ochre because ofits manganese and iron oxide content. It is highiyvaiued as a pe「manent pigmenteither in the raw or burntstate. Umber is Iightfast, insoluble in water, 「eSistantto alkaIis and weak acids and non-reaCtive with cement, SOIvents, OiIs, and most resins. -

A Color Mixing Guide. CREA Your Color Story with Colors You Practice in Advance and Love
Use the color cards in this kit as an optional tool as you create. Fill it up with favorite color blends as you work ORY through the workshop (and notes on OLOR ST TE A C how to achieve them again), or pre-plan A color mixing guide. CREA your color story with colors you practice in advance and love. The best way to design beautiful A quick intro to figuring out colors. color schemes is to play! I’m so excited and glad that you’ve decided to paint with me in this workshop. I really hope the information in this booklet is helpful. Don’t be afraid to outsource for further information, Pinterest is a great tool for color schemes, and you can find just about everything else on Google. Enjoy yourself and have fun! THE THE CRAFTER’S CRAFTER’S BOX BOX Mixing paint doesn’t have to be hard! Let’s be honest, mixing colors seems so complicated. I hear that all the time. And anyone who has tried their hand at blending colors and has ended up with several different shades of brown by accident knows what I’m saying. I’m hoping this guide will help walk you though this workshop and also give you the confidence to blend many different shades of brown on purpose. I should also mention that while I feel very knowledgeable about color theory in the application I use it, I’m not actually a master. If this guide isn’t helpful or is conflicting to your college level color theory course, feel free to gently place this in the trash. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Deep Olive Speckle
PC-1 Saturation Cone 5-6 Metallic* over PC-29 Deep Olive Speckle PC-4 PC-25 PC-30 Palladium Textured Temmoku over Turquoise over Layering PC-29 over PC-29 Deep Olive PC-29 Deep Olive Speckle Deep Olive Speckle Speckle PC-12 PC-27 PC-32 Blue Midnight Tourmaline Albany Slip over over Brown* PC-29 PC-29 over Deep Olive Deep Olive PC-29 Speckle Speckle Deep Olive Speckle PC-23 PC-28 PC-33 Indigo Float Frosted Iron Lustre over Turquoise over PC-29 over PC-29 Deep Olive PC-29 Deep Olive Speckle Deep Olive Speckle Speckle PC-29 Layering Notes: Brush on two Cups: Josh Heim over PC-34 layers of base coat. Brush on two layers Clay: AMACO® Buff Stoneware Clay No. 46 Light Sepia of top coat. (Let dry between coats.) Firing: Test tiles have been fired to Cone 6 over PC-29 www.layeringpcs.info PC-29 Deep Olive Deep Olive Speckle Speckle Join the conversation www.facebook.com/groups/potterschoiceex vary may Color between printed copies. PC-35 PC-41 PC-52 Oil Spot Vert Lustre Deep Sienna over over Speckle PC-29 PC-29 over Deep Olive Deep Olive PC-29 Speckle Speckle Deep Olive Speckle PC-36 PC-42 PC-53 Ironstone Seaweed Ancient Jasper over over over PC-29 PC-29 PC-29 Deep Olive Deep Olive Deep Olive Speckle Speckle Speckle PC-37 PC-43 PC-55 Smoked Toasted Sage Chun Plum Sienna over over over PC-29 PC-29 PC-29 Deep Olive Deep Olive Deep Olive Speckle Speckle Speckle PC-39 PC-46 PC-59 Umber Float Lustrous Jade Deep over over Firebrick PC-29 PC-29 over Deep Olive Deep Olive PC-29 Speckle Speckle Deep Olive Speckle The Art & Creative Materials Institute AP (Approved PC-49 PC-60 Product) seal certifies this product to be safe for use Frosted Melon Salt Buff* by all ages. -

Textures of Williamsburg Handmade Oil Colors
Textures of Williamsburg Handmade Oil Colors VERY FINE FINE MEDIUM COARSE Alizarin Orange Permanent Red-Orange Brown Umber Alizarin Crimson Brown Pink Bismuth Vanadate Yellow Permanent Yellow Deep Burnt Sienna Alizarin Yellow Dutch Brown (Transparent) Brilliant Yellow Extra Pale Permanent Yellow Light Burnt Umber Bohemian Green Earth Italian Pink Brilliant Yellow Pale Permanent Yellow Medium Cadmium Green Brown Ochre Olive Green Carl’s Crimson Persian Rose Cadmium Green Light Cyprus Orange Stil De Grain Cerulean Blue French Phthalo Blue Cadmium Lemon Earth Green Chromium Oxide Green Phthalo Green Cadmium Orange French Ardoise Grey Cinnabar Green Light Phthalo Green-Yellowish Cadmium Purple French Brown Ochre Cobalt Blue Phthalo Turquoise Cadmium Red Deep French Burnt Ochre Cobalt Blue Deep Provence Violet Bluish Cadmium Red Light French Burnt Umber Cobalt Green Provence Violet Reddish Cadmium Red Medium French Light Sienna Cobalt Teal Bluish Prussian Blue Cadmium Red Purple French Ochre Havane Cobalt Teal Greenish Pyrrole Orange Cadmium Red Vermilion French Raw Sienna Cobalt Turquoise Bluish Pyrrole Red Cadmium Yellow Deep French Raw Umber Cobalt Turquoise Greenish Quinacridone Magenta Cadmium Yellow Extra Deep French Rouge Indien Cobalt Violet Deep Quinacridone Red Cadmium Yellow Light French Terre Verte Cobalt Violet Light Quinacridone Violet Cadmium Yellow Medium French Yellow Ochre Deep Courbet Green Sevres Blue Canton Rose German Earth Dianthus Pink SF Cerulean Blue French Cerulean Blue (Genuine) Graphite Grey Egyptian Violet SF -

Paint Pigments— Yellow
» TECHNICAL INFORMATION ON BUILDING MATERIALS TIBM - 32 FOR UfSE IN THE DESIGN OF LOW-COST HOUSING ***** THE NATIONAL BUREAU OF STANDARDS UNITED STATES DEPARTMENT OF COMMERCE WASHINGTON, D. C. August 29, 1936 PAINT PIGMENTS— YELLOW, . BROWN, BLUE, GREEN, AND BRONZE This is urimarily^a digest of the sections of Bureau of Standards Circular No, o9> "Paint and Varnish", (November 17, 1917),'*' and Tech- nologic Paper No. 274, "Use of United States Government Suecif ication Paints and Faint Materials", (December 15, 1924), ^ Ly p, H. Walker and E. F. Hickson, dealing with general composition , characteristics, and uses of yellow, brown, blue, green, and bronze pigments. The following papers contain additional information relative to paint pigments, oil paints, and water paints: TIBM - 30 "Paint Pigments—White" TIBM - 31 "Paint Pigments—Black, Red, and Lakes" TIBM - 33 "Federal Specification . Paint Pigments and Mixing Formulas" TIM - 3U "Federal Specification Ready-Mixed Paints, Semi- paste Paints and Mixing Formulas’"' TIBM - 35 "Preparation of Paints from Paste and Dry Pigments" TIBM - 36 "Preparation of Paints from Semipaste Paints, Thinning Ready-Mixed Paints, and Preparation of Water Paints" TIBM - 43 "Aluminum Paints" Pigments are "the fine solid warticles used in the preparation of paint, and substantially insoluble in the vehicle, "3 In general, it may be ^Out of print. May be consulted in Government depositor}*- libraries. p Available from Superintendent of Documents, Government Printing Office, Washington, D. C. .(Price 10 cents). ^Qpioted from "Standard Definitions of Terms Relating to Paint 'Specifications", American Society for Testing Materials ( 1 93 3 ) ’ • • -• •• PP. 735-73 9 . 031736-C - 1 - assumed that pigments composed of very fine particles, having high re- fractive indices, provide the greatest covering power and opacity. -
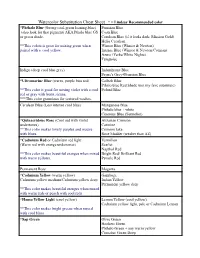
Watercolor Substitution Cheat Sheet * = Lindsay Recommended Color
Watercolor Substitution Cheat Sheet * = Lindsay Recommended color *Phthalo Blue (Strong cool-green leaning-blue) Prussian Blue (also look for that pigment) AKA Pthalo blue GS Cyan Blue or green shade. Cerulean Blue (if it looks dark: Mission Gold) Helio Cerulean **This colors is great for mixing green when Winsor Blue (Winsor & Newton) paired with a cool yellow. Intense Blue (Winsor & Newton/Cotman) Azure (Yarka/White Nights) Turquoise Indigo (deep cool blue grey) Indanthrone Blue Payne's Grey+Prussian Blue *Ultramarine Blue (warm, purple bias red) Colbalt Blue Pthalo blue Red Shade (not my fave substitute) **This color is good for mixing violet with a cool Poland Blue red or gray with burnt sienna. ***This color granulates for textured washes. Cerulean Blue (Less intense cool blue) Manganese Blue Phthalo blue + white Cinerous Blue (Sennelier) *Quinacridone Rose (Cool red with violet Alizarian Crimson undertones) Carmine **This color makes lovely purples and mauve Crimson lake with blues. Rose Madder (weaker than AZ) *Cadmium Red or Cadmium red light Vermilion (Warm red with orange undertones) Scarlet Napthol Red **This color makes beautiful oranges when mixed Bright Red/ Brilliant Red with warm yellows. Pyrrole Red Permanent Rose Magenta *Cadmium Yellow (warm yellow) Gamboge Cadmium yellow medium/Cadmium yellow deep Indian Yellow Permanent yellow deep **This color makes beautiful oranges when mixed with warm reds or peach with cool reds *Hansa Yellow Light (cool yellow) Lemon Yellow (cool yellow) Cadmium yellow light, pale or Cadmium -

Glossary of Obsolete Mineral Names
Uaranpecherz = uraninite, László 282 (1995). überbasisches Cuprinitrat = gerhardtite, Hintze I.3, 2741 (1916). überbrannter Amethyst = heated 560ºC red-brown Fe-rich quartz, László 11 (1995). Überschwefelblei = galena + anglesite + sulphur-α, Chudoba RI, 67 (1939); [I.3,3980]. uchucchacuaïte = uchucchacuaite, MR 39, 134 (2008). uddervallite = pseudorutile, Hey 88 (1963). uddevallite = pseudorutile, Dana 6th, 218 (1892). uddewallite = pseudorutile, Des Cloizeaux II, 224 (1893). udokanite = antlerite, AM 56, 2156 (1971); MM 43, 1055 (1980). uduminelite (questionable) = Ca-Al-P-O-H, AM 58, 806 (1973). Ueberschwefelblei = galena + anglesite + sulphur-α, Egleston 132 (1892). Uekfildit = wakefieldite-(Y), Chudoba EIV, 100 (1974). ufalit = upalite, László 280 (1995). uferite = davidite-(La), AM 42, 307 (1957). ufertite = davidite-(La), AM 49, 447 (1964); 50, 1142 (1965). U-free thorite = huttonite, Clark 303 (1993). U-galena = U-rich galena, AM 20, 443 (1935). ugandite = bismutotantalite, MM 22, 187 (1929). ughvarite = nontronite ± opal-C, MAC catalog 10 (1998). ugol = coal, Thrush 1179 (1968). ugrandite subgroup = uvarovite + grossular + andradite ± goldmanite ± katoite ± kimzeyite ± schorlomite, MM 21, 579 (1928). uhel = coal, Thrush 1179 (1968). Uhligit (Cornu) = colloidal variscite or wavellite, MM 18, 388 (1919). Uhligit (Hauser) = perovskite or zirkelite, CM 44, 1560 (2006). U-hyalite = U-rich opal, MA 15, 460 (1962). Uickenbergit = wickenburgite, Chudoba EIV, 100 (1974). uigite = thomsonite-Ca + gyrolite, MM 32, 340 (1959); AM 49, 223 (1964). Uillemseit = willemseite, Chudoba EIV, 100 (1974). uingvárite = green Ni-rich opal-CT, Bukanov 151 (2006). uintahite = hard bitumen, Dana 6th, 1020 (1892). uintaite = hard bitumen, Dana 6th, 1132 (1892). újjade = antigorite, László 117 (1995). újkrizotil = chrysotile-2Mcl + lizardite, Papp 37 (2004). új-zéalandijade = actinolite, László 117 (1995). -

A Study of Unusual Black and Dark Grey Pigments Used by Artists in the Sixteenth Century
National Gallery Technical Bulletin Volume 24, 2003 National Gallery Company London Distributed by Yale University Press This volume of the Technical Bulletin is published with the generous support of the Samuel H. Kress Foundation and the American Friends of the National Gallery, London, Inc. Series editor Ashok Roy © National Gallery Company Limited 2003 All rights reserved. No part of this publication may be transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without the prior permission in writing of the publisher. First published in Great Britain in 2003 by National Gallery Company Limited St Vincent House, 30 Orange Street London wc2h 7hh www.nationalgallery.co.uk British Library Cataloguing in Publication Data A catalogue record for this journal is available from the British Library isbn 1 85709 997 4 issn 0140 7430 525043 Publisher Kate Bell Project manager Jan Green Editor Diana Davies Designer Tim Harvey Picture research Xenia Corcoran and Kim Klehmet Production Jane Hyne and Penny Le Tissier Printed in Italy by Conti Tipocolor front cover Georges Seurat, Bathers at Asnières (NG 3908), detail of plate 4, page 7 title page Giulio Romano, The Birth of Jupiter (NG 624), detail of plate 1, page 38 ‘Black Earths’: A Study of Unusual Black and Dark Grey Pigments used by Artists in the Sixteenth Century marika spring, rachel grout and raymond white n his Lives of the Artists, Vasari describes ‘an beautiful for all lights and all shadows, and espe- Iastounding piece of painting’ by Sebastiano del cially if it is the one from Venice, which comes in Piombo, a portrait of Pietro Aretino in which there little balls’.8 A terra nera di campana (black earth of may be seen ‘five or six different kinds of black in bells) is mentioned by Vasari, Armenini, Lomazzo, the clothes that he is wearing – velvet, satin, Borghini and Baldinucci. -

41991 Earth Color Assortment
41991 Earth Color Assortment Approx. 70 earth colors packed in clear jars, 50 g each. Contents (depending on availability) 40010 - French Ochre JTCLES 40520 - Red Bole in pieces 40012 - French Ochre, very light 40542 - English Red Light 40020 - French Ochre RTFLES 40545 - English Red Deep 40030 - French Ochre JOLES 40610 - Raw Umber, from Cyprus 40040 - French Ochre JCLES 40611 - Raw Umber, light 40050 - French Ochre JFLES 40612 - Raw Umber, Italy 40060 - French Ochre JALS 40623 - Manganese Brown Intense 40070 - French Ochre SOFODOR 40630 - Raw Umber greenish dark 40080 - French Ochre HAVANE 40650 - Chrome-Iron Stone 40090 - French Ochre SOFOROUGE 40660 - Raw Umber dark 40130 - French Ochre SAHARA 40690 - Umber Reddish, OK 46 40194 - Gold Ochre 40700 - Burnt Umber Reddish 40200 - Ochre Avana, greenish-yellow 40710 - Burnt Umber, Cyprus 40210 - Gold Ochre H 84 40720 - Burnt Umber, Cyprian, dark brown 40214 - Gold Ochre DD 40723 - Burnt Umber type B 40220 - Italian Gold Ochre light 40730 - Burnt Umber light reddish-brown 40231 - Brown Ochre 40800 - Green Earth, German 40241 - Fawn Ochre, German 40810 - Bohemian Green Earth 40260 - Satin Ochre, Monte Amiata 40821 - Green Earth from Verona 40280 - Amberg Yellow 40830 - Green Earth from France 40301 - Iron Oxide Yellow 40850 - Green Earth, burnt 40310 - Dark Ochre, German 40900 - Slate Gray, extra light 40320 - Dark Ochre, Italian 40911 - Slate Gray, greenish 40351 - Red Mine Ochre 40920 - Slate Gray, gray-green 40391 - Raw Sienna from England 40960 - Pencil Clay, 0 - 0.5 mm 40392 - Raw Sienna, French 40970 - Pencil Clay, pieces 40400 - Raw Sienna, Italy 41000 - Van Dyck Brown 40404 - Raw Sienna Badia, Italy 41050 - Cassel brown, wood stain 40410 - Raw Sienna brownish, Italy 41550 - Terra Pozzuoli 40430 - Burnt Sienna No.