Lakes a to Z Help Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scottsdale Arabian Horse Show Feb 16 2017 Result Back# Horse Name
Results by Class: Scottsdale Arabian Horse Show Feb 16 2017 Result Back# Horse Name / Sire x Dam Owner / Breeder Handler Page: 1 Class#: 1G Arabian Hunter Pleasure JTR 17-18yrs 16 Shown Judge:Terri Delbridge Jody LaSalle Lisa Jo White 1 1071 C HONDO Wolf Springs Ranches Inc Anna Redmond ENZO X ENCHANTES BEY Bill Cunningham 2 1685 JUSTTINIAN Lauren Ambrosini Lauren Ambrosini JUSTIFY X SHAHMAICA BEY Robert C Or Janene M Boggs 3 1871 REDEMPTION H Alyssa or Marlene Tomusiak Alyssa Tomusiak MILLENNIUM LOA X JORDONA Peggy Heathcott 4 1030 SF AHEIR OVERDRESSED Michelle Kurth or Tiffany Kurth Madison Schwanz AFIRES HEIR X SF ALEXIS Springwater Farms Arabians LLC 5 284 PALADIN LL LMJ Investments LLC Julia Nastri MAGNUM CHALL HVP X NV GYPSY Leslie A Lurken DANCER 6 1972 CP GUNCYN ROSES Kirsten Luedde Kirsten Luedde CYTOSK X BARBS MIST Cal Poly Pomona 7 1750 KAYCEE OF JEWYLLS LAS Kathryn M Schwartz Abigail Buschette SHOWKAYCE X KHNIGHT JEWYLL LAS Lavoy Shepherd 8 1442 JUSTA HOOT Bailey and Jessica Mirmelli Bailey Mirmelli JUSTAFIRE DGL X FSR BARBARA Michael J or Victoria J Mangan 9 1759 ATLANTIS SC Charles or Christine or Adam Rickart Mikenna Laventure ODYSSEY SC X CARISMRETA Sparkle Creek LLC 10 753 KYROS ROMEO Wendy or Kaitlyn Ruonavaara Kaitlyn Ruonavaara DA VALENTINO X SR MAGIC MERCY Charlene L Strong Class#: 1H Arabian Hunter Pleasure JTR 15-16yrs 16 Shown Judge:Terri Delbridge Jody LaSalle Lisa Jo White 1 1043 DA SOVEREIGN Emma or Jody Freeland Emma Freeland SUNDANCE KID V X DA FAITH Dolorosa Arabians LTD 2 1881 ARAMIS LBA+ Ali Brady and Matthew -
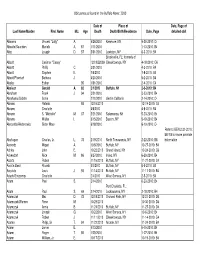
Obituaries Buffalo News 2010 by Name
Obituaries as found in the Buffalo News: 2010 Date of Place of Date, Page of Last Name/Maiden First Name M.I. Age Death Death/Birth/Residence Date, Page detailed obit Abbarno Vincent "Lolly" A. 9/26/2010 Kenmore, NY 9-30-2010: C4 Abbatte/Saunders Murielle A. 87 1/11/2010 1-13-2010: B4 Abbo Joseph D. 57 5/31/2010 Lewiston, NY 6-3-2010: B4 Brooksville, FL; formerly of Abbott Casimer "Casey" 12/19/22009 Cheektowaga, NY 4-18-2010: C6 Abbott Phillip C. 3/31/2010 4-3-2010: B4 Abbott Stephen E. 7/6/2010 7-8-2010: B4 Abbott/Pfoetsch Barbara J. 4/20/2010 5-2-2010: B4 Abeles Esther 95 1/31/2010 2-4-2010: C4 Abelson Gerald A. 82 2/1/2010 Buffalo, NY 2-3-2010: B4 Abraham Frank J. 94 3/21/2010 3-23-2010: B4 Abrahams/Gichtin Sonia 2/10/2010 died in California 2-14-2010: C4 Abramo Rafeala 93 12/16/2010 12-19-2010: C4 Abrams Charlotte 4/6/2010 4-8-2010: B4 Abrams S. "Michelle" M. 37 5/21/2010 Salamanca, NY 5-23-2010: B4 Abrams Walter I. 5/15/2010 Basom, NY 5-19-2010: B4 Abrosette/Aksterowicz Sister Mary 6/18/2010 6-19-2010: C4 Refer to BEN 2-21-2010: B6/7/8 for more possible Abshagen Charles, Jr. L. 73 2/19/2010 North Tonawanda, NY 2-22-2010: B8 information Acevedo Miguel A. 10/6/2010 Buffalo, NY 10-27-2010: B4 Achkar John E. -

Incidents of Lawlessness- Tileodore Roosevelt Bags His
Incidents of Lawlessness and expense of keeping our convicts ...The nun1ber is increasing and Tileodore Roosevelt Bags His Men with our largely increasing population it will continue to increase."1 During the 1880's in western Dakota, lawbreakers were certainly by Dr. Aaron Woodard a feature of life. One account described the area as follows, ''Little Missouri was a terrible place, .. .it was wild and wooly... there were n1any of them, outcasts of society, reckless, greedy and conscienceless; One feature of the Old West that is known to almost everyone is fugitives fron1 justice with criminal records and gunn1en who lived the role of the outlaw and the lawman. A staple of western 1novies by crooked ga~nbling and thievery of every sort."2 There were also and novels, this duel between good and evil figures prominently into brutal murders in Dakota, including the case of George Miller, who American culture-then and now. Many modern "Americanisms" have murdered a Baptist minister and his 6 year old daughter with an axe their beginning in this struggle for law and order in the West. The classic and knife in 1885. There was also the 1894 case, shortly after North showdown at high noon in some dusty western town between a lone Dakota statehood, of Albert Bomberger who murdered six members sheriff or marshal and a gang of cutthroats remains a popular topic for of the same family after he was apparently denied the chance to westerns but also, in a somewhat modified form, for modern action films. engage his romantic intentions towards the youngest daughter of the family. -

Dau's Blue Book 1908
Central Library of Rochester and Monroe County · Miscellaneous Directories Central Library of Rochester and Monroe County · Miscellaneous Directories Rochester Tels., 3333-5933 Bell, 2912 Main Electric Lighting Electric, Gas and Combination Fixtures, Art Mosaics. Table Lamps. Lighting Novelties. Welsbach Repairs. Electric Wiring for Light, Heat and Power Transmission. Bell and Telephone Installation and Repairs Prompt and efficient Rochester Public Library Reference Book Not For Circulation ABNER . ^X^Nl J ''ADAM S Uontractor Fine Hardwood Floors Contracts made for hardwood floors of every approved thickness and style, laid and finished complete in old or new houses Western Ne-iv York Agency Wood-Mosaic Flooring Company 29 East Avenue Rochester, N. Y. Central Library of Rochester and Monroe County · Miscellaneous Directories 3 9077 05345 8660 Choice f loftier?, "25CUNTONAVE.,N>S 2Decoration£, $alms, FROCH|2EJ^L f c™$ ™* f lotomng plants Home Phone IQ48 Bell Phone 666 Mai. Ereb ©• ©tcfeforb Storage and Manufacturing Co. AWNINGS, SANITARY BED- DING & UPHOLSTERING Furniture & Merchandise Moved Packed & Shipped Celebrated Nantucket The only First-class Storage Hammocks House in the City 359-373 State Street Rochester, N. 2 12-18 Frankfort Street Central Library of Rochester and Monroe County · Miscellaneous Directories HOME PHONE 2367 BELL MAIN 594-Y Creamy T% /f¥T \f Absolutely and Rich MILK\/l I I Iv. No Sediment KEPT SCRUPULOUSLY CLEAN FROM THE HEALTHY COW TO OUR STER ILIZED BOTTLE Hay den Bros. Milk Co. 574 West Avenue ESTABLISHED 1882 HOME PHONE 287 INCORPORATED 1906 BELL PHONE 121 CHASE Cayford C& Sons Livery Co. Coaches, Coupes and all Kinds of Livery 30 and 32 N. -

The Sisters in Crime Newsletter Volume XXII • Number 3 September 2009
IInnSSiinnCC The Sisters in Crime Newsletter Volume XXII • Number 3 September 2009 SinC Puts Forward StroPnick. MgarySis anlawaardt- eat tfheoRefreren2ce D0esk0and9as -the2lib0rary1’s PR0 co - ByCRlicohébyeers,tabuItsitletriubl,y PseeamsstlPikereyessitderednayt that winning poet and short ordinator. One of the very best parts of her job is Libby Hellmann called to ask if I’d consider tak - story writer and former bringing writers — especially mystery writers — ing a spot on the Sisters in Crime National Board recording engineer. She to the library. “There is absolutely nothing better of Directors. These four years have been chal - lives in Nashville. than for a library user to tell me they have been lenging and so very rewarding and it’s with mixed Treasurer Kathryn reading a mystery author because they met her at feelings that I step down. On behalf of the nomi - R. Wall is the author of the library and that they can’t wait for her next nating committee, I am delighted to offer SinC Covenant Hall and book to come out!” members a very strong slate for the 2009-2010 eight previous Bay Tan - Nancy Martin board. Elections will take place at Bouchercon in ner mysteries set in the (Member At Large) Indiana. If you’re not planning to attend, please Mary Saums Low Country of South wrote more than 40 ro - vote by mail, using the ballot on page 15 in this is - Carolina. The Mercy mance novels before sue. And now, the slate: Oak , released in 2008, turning to her real pas - President-elect was a Southeastern In - sion – murder myster - Marcia Talley is the dependent Book - ies. -

Greenville, MS 38702-1873 with Your Continued Service to Those in Need, Especially the Children
NATIONAL ASSOCIATION OF JUNIOR AUXILIARIES, INC. 845 South Main Street Greenville, Mississippi 38701 Mailing Address: Post Office Box 1873 Greenville, Mississippi 38702-1873 Telephone: (662) 332-3000 Facsimile: (662) 332-3076 E-Mail: [email protected] Web site: www.najanet.org Facebook: https://www.facebook.com/NAJAinc Follow us on Twitter: najainc Pinterest: http://pinterest.com/najainc/ LinkedIn: http://www.linkedin.com/groups/National-Association-Junior-Auxiliaries-Inc-4502392 The Junior Auxiliary membership list provided in this Bulletin is for the use of the Junior Auxiliary only and cannot be used for promotion of any activity unrelated to the Junior Auxiliary. “The Junior Auxiliary membership list shall not be made available for commercial purposes or for the purpose of solicitation.” Association Standing Rules I. Association. C. Fund Raising and Contributions. Junior Auxiliary Prayer Send us, O God, as Thy messengers to the hearts without a home, to lives without love, to the crowds without a guide. Send us to the children whom none have blessed, to the famished whom none have visited, to the fallen whom none have lifted, to the bereaved whom none have comforted. Kindle Thy flame on the altars of our hearts, that others may be warmed thereby; cause Thy light to shine in our souls, that others may see the way; keep our sympathies and insight ready, our wills keen, our hands quick to help others in their need. Grant us clear vision, true judgment, with great daring as we seek to right the wrong; and so endow us with cheer- ful love that we may minister to the suffering and forlorn even as Thou wouldst. -

Hold the Dark 2018
HOLD THE DARK based on the novel by William Giraldi written for the screen by Macon Blair 12.10.15 A24 / VisionChaos Productions / Addictive Pictures Bonneville Films THIS APPEARS ON BLACK SCREEN: O unteachably after evil, but uttering truth. - Gerald Manley Hopkins EXT. THE ALASKAN FRONTIER - DAY SUNLIGHT, as bright and sharp as a blade, slicing down on this moonscape of SNOW and ICE. Feel the cold in your teeth. The wind makes a faint flute-sound and all else is silence. In the distance sit god-sized hills, hazy and dream-like. And spread before them, abutting a black bristle of trees... A VILLAGE. Just a handful of buildings, looking like toy blocks dropped onto a clean white quilt. SUPER: Keelut, Alaska. How do people even survive out here? And why? EXT. BEHIND THE SLONE CABIN - DAY Not so much a backyard as an expanse between the distant TREELINE and a roughly-hewn LOG CABIN, smoking chimney and mossy walls, a soft rhythmic wump wump wump leading us to... A BOY (7), kneeling to pack a small mound of snow. Wump, wump, wump. He looks swollen in his pillowed coat, working a well-used ARMY MAN into the mound. His name is BAILEY. His nose running, his breath clouding before his face, he’s wholly focused on this very serious work until... He looks up. Sees something. Freezes. A WOLF. Just emerged from the trees. Motionless. Yellow eyes fixed on Bailey, her own breath steaming. She’s huge. ON BAILEY, seen from behind, looking out at the WOLF, only forty feet away. -

Commencement Exercises
COMMENCEMENT EXERCISES SPRING / WINTER 2020 TABLE OF CONTENTS Welcome ................................................................................................................... 2 Greetings from the Board of Visitors ........................................................................ 3 Greetings from the Alumni Association .................................................................... 4 History of Towson University ................................................................................... 6 University Traditions ..............................................................................................10 Ceremony Etiquette ................................................................................................ 13 Event Information ..................................................................................................14 Grand Marshals ...................................................................................................... 16 Commencement Speaker ........................................................................................20 Commencement Student Speakers ..........................................................................22 Honors College ....................................................................................................... 26 College of Health Professions Overview ................................................................................................................28 Order of Exercises.. ................................................................................................30 -

Charted Lakes List
LAKE LIST United States and Canada Bull Shoals, Marion (AR), HD Powell, Coconino (AZ), HD Gull, Mono Baxter (AR), Taney (MO), Garfield (UT), Kane (UT), San H. V. Eastman, Madera Ozark (MO) Juan (UT) Harry L. Englebright, Yuba, Chanute, Sharp Saguaro, Maricopa HD Nevada Chicot, Chicot HD Soldier Annex, Coconino Havasu, Mohave (AZ), La Paz HD UNITED STATES Coronado, Saline St. Clair, Pinal (AZ), San Bernardino (CA) Cortez, Garland Sunrise, Apache Hell Hole Reservoir, Placer Cox Creek, Grant Theodore Roosevelt, Gila HD Henshaw, San Diego HD ALABAMA Crown, Izard Topock Marsh, Mohave Hensley, Madera Dardanelle, Pope HD Upper Mary, Coconino Huntington, Fresno De Gray, Clark HD Icehouse Reservior, El Dorado Bankhead, Tuscaloosa HD Indian Creek Reservoir, Barbour County, Barbour De Queen, Sevier CALIFORNIA Alpine Big Creek, Mobile HD DeSoto, Garland Diamond, Izard Indian Valley Reservoir, Lake Catoma, Cullman Isabella, Kern HD Cedar Creek, Franklin Erling, Lafayette Almaden Reservoir, Santa Jackson Meadows Reservoir, Clay County, Clay Fayetteville, Washington Clara Sierra, Nevada Demopolis, Marengo HD Gillham, Howard Almanor, Plumas HD Jenkinson, El Dorado Gantt, Covington HD Greers Ferry, Cleburne HD Amador, Amador HD Greeson, Pike HD Jennings, San Diego Guntersville, Marshall HD Antelope, Plumas Hamilton, Garland HD Kaweah, Tulare HD H. Neely Henry, Calhoun, St. HD Arrowhead, Crow Wing HD Lake of the Pines, Nevada Clair, Etowah Hinkle, Scott Barrett, San Diego Lewiston, Trinity Holt Reservoir, Tuscaloosa HD Maumelle, Pulaski HD Bear Reservoir, -

Republican Journal: Vol. 65, No. 32
The Republican Journal. Mi: l’> HI. FA ST, MAINE TillRSHA V, UT.IST 1<>, ISiKL NLMBLrTj^ names "1 nun imp. mated in the affair. Tons of Water watching it seemed that the horse dashed Personal. Personal. Nortliport ( amp ('.round and Vicinity. 'publican Journal. A : M-A.ifd bah «-ny at the house through the torrent, dragging Conley the lu ,m-,i Vaeht hub rave Lt-t BI'IJST ITeN TII EI it H A I’I.ESS HHAI*s. A < way Hhers think that the two Mrs. Lizzie M. Beckwith m v s ta fra ids along. escaped Mrs. Kusseli Brier is relatives at g Id" k.and l.a of d. \V. S M K M M. HA 1 K Thursday evening pi .-mpitatine a nuni- EAMII Y IN A MOMENT SWEPT To I'EATII. while himself thinks visiting partus Fought independently Conley Fort in Windsor ''ei .a to ami A I' 1MEIT UlSiOYKliY SAVES Point. *mbs Tli*- 1’avilioi: property on South peoj-lt the pui into the wa- ANOIHKK that a hencoop which he clutched, buoyed ter. Killeen or were AM11.V. A HI Km IHE> WHILE ENf. A»«- •Lillies is at home fr- n: B«*st« i. f.-r Sl.or** and wf.i -ins simmm. dc Journal Co. twenty hurt, and J. him until he found safety. At any rate I Mr Wilson Carter returned to Camden by Fogarty manage < 1 Fnlilisliina 1'. Warm h adei t the iielsea band, A. EU IN I!E>( INI-. DEVASTATION EKoM came a vacation. he out on the north side tor- of the Saturday's boat. -

2008 International List of Protected Names
LISTE INTERNATIONALE DES NOMS PROTÉGÉS (également disponible sur notre Site Internet : www.IFHAonline.org) INTERNATIONAL LIST OF PROTECTED NAMES (also available on our Web site : www.IFHAonline.org) Fédération Internationale des Autorités Hippiques de Courses au Galop International Federation of Horseracing Authorities _________________________________________________________________________________ _ 46 place Abel Gance, 92100 Boulogne, France Avril / April 2008 Tel : + 33 1 49 10 20 15 ; Fax : + 33 1 47 61 93 32 E-mail : [email protected] Internet : www.IFHAonline.org La liste des Noms Protégés comprend les noms : The list of Protected Names includes the names of : ) des gagnants des 33 courses suivantes depuis leur ) the winners of the 33 following races since their création jusqu’en 1995 first running to 1995 inclus : included : Preis der Diana, Deutsches Derby, Preis von Europa (Allemagne/Deutschland) Kentucky Derby, Preakness Stakes, Belmont Stakes, Jockey Club Gold Cup, Breeders’ Cup Turf, Breeders’ Cup Classic (Etats Unis d’Amérique/United States of America) Poule d’Essai des Poulains, Poule d’Essai des Pouliches, Prix du Jockey Club, Prix de Diane, Grand Prix de Paris, Prix Vermeille, Prix de l’Arc de Triomphe (France) 1000 Guineas, 2000 Guineas, Oaks, Derby, Ascot Gold Cup, King George VI and Queen Elizabeth, St Leger, Grand National (Grande Bretagne/Great Britain) Irish 1000 Guineas, 2000 Guineas, Derby, Oaks, Saint Leger (Irlande/Ireland) Premio Regina Elena, Premio Parioli, Derby Italiano, Oaks (Italie/Italia) -

2009 International List of Protected Names
Liste Internationale des Noms Protégés LISTE INTERNATIONALE DES NOMS PROTÉGÉS (également disponible sur notre Site Internet : www.IFHAonline.org) INTERNATIONAL LIST OF PROTECTED NAMES (also available on our Web site : www.IFHAonline.org) Fédération Internationale des Autorités Hippiques de Courses au Galop International Federation of Horseracing Authorities __________________________________________________________________________ _ 46 place Abel Gance, 92100 Boulogne, France Tel : + 33 1 49 10 20 15 ; Fax : + 33 1 47 61 93 32 E-mail : [email protected] 2 03/02/2009 International List of Protected Names Internet : www.IFHAonline.org 3 03/02/2009 Liste Internationale des Noms Protégés La liste des Noms Protégés comprend les noms : The list of Protected Names includes the names of : ) des gagnants des 33 courses suivantes depuis leur ) the winners of the 33 following races since their création jusqu’en 1995 first running to 1995 inclus : included : Preis der Diana, Deutsches Derby, Preis von Europa (Allemagne/Deutschland) Kentucky Derby, Preakness Stakes, Belmont Stakes, Jockey Club Gold Cup, Breeders’ Cup Turf, Breeders’ Cup Classic (Etats Unis d’Amérique/United States of America) Poule d’Essai des Poulains, Poule d’Essai des Pouliches, Prix du Jockey Club, Prix de Diane, Grand Prix de Paris, Prix Vermeille, Prix de l’Arc de Triomphe (France) 1000 Guineas, 2000 Guineas, Oaks, Derby, Ascot Gold Cup, King George VI and Queen Elizabeth, St Leger, Grand National (Grande Bretagne/Great Britain) Irish 1000 Guineas, 2000 Guineas,