Chriacus: New Insight Into the Neurosensory System and Evolution of Early Placental Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Paleocene Mammalian Faunas of the Bison Basin in South-Central Wyoming
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 131, NUMBER 6 Cftarlesi ©, anb JHarp ^aux SKHalcott 3Res!earcf) Jf unb PALEOCENE MAMMALIAN FAUNAS OF THE BISON BASIN IN SOUTH-CENTRAL WYOMING (With 16 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology United States National Museum Smithsonian Institution (Publication 4229) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION FEBRUARY 28, 1956 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction i Acknowledgments 2 History of investigation 2 Occurrence and preservation of material 3 The Bison basin faunas 4 Environment and relationships between the Bison basin faunas 7 Age and correlation of the faunas lo Systematic description of vertebrate remains I2 Reptilia 12 Sauria 12 Anguidae 12 Mammalia 12 Multitubcrculata 12 Ptilodontidae 12 Marsupialia 14 Didelphidae 14 Insectivora 15 Leptictidae 15 Pantolestidae 17 Primates 19 Plesiadapidae 19 Carnivora 25 Arctocyonidae 25 Mesonychidae 35 Miacidae 35 Condylarthra 36 Hyopsodontidae 36 Phenacodontidae 42 Pantodonta 47 Coryphodontidae 47 References 51 Explanation of plates 54 ILLUSTRATIONS Plates (All plates following page 58.) 1. Mullituberculates and insectivores from the Bison basin Paleocene. 2. Primates and marsupials from the Bison basin Paleocene. 3. Pronothodcctes from the Bison basin Paleocene. 4. Plcsiadapis from the Bison basin Paleocene. 5. Triccntcs and Chriacus from the Bison basin Paleocene. 6. Thryptacodon from the Bison basin Paleocene. 7. Clacnodon from the Bison basin Paleocene. 8. Litomylus and Protoselcnc? from the Bison basin Paleocene. 9. Haplaletes and Gidleyina from the Bison basin Paleocene. 10. Phcnacodusl from the Bison basin Paleocene. 11. Condylarths and Titanoidcs from the Bison basin Paleocene. 12. Caenolambda from the Bison basin Paleocene. -

Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology
JOURNAL OF MORPHOLOGY 277:1469–1485 (2016) A Three-Dimensional Morphometric Analysis of Upper Forelimb Morphology in the Enigmatic Tapir (Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology Jamie A. MacLaren1* and Sandra Nauwelaerts1,2 1Department of Biology, Universiteit Antwerpen, Building D, Campus Drie Eiken, Universiteitsplein, Wilrijk, Antwerp 2610, Belgium 2Centre for Research and Conservation, Koninklijke Maatschappij Voor Dierkunde (KMDA), Koningin Astridplein 26, Antwerp 2018, Belgium ABSTRACT Forelimb morphology is an indicator for order Perissodactyla (odd-toed ungulates). Modern terrestrial locomotor ecology. The limb morphology of the tapirs are widely accepted to belong to a single enigmatic tapir (Perissodactyla: Tapirus) has often been genus (Tapirus), containing four extant species compared to that of basal perissodactyls, despite the lack (Hulbert, 1973; Ruiz-Garcıa et al., 1985) and sev- of quantitative studies comparing forelimb variation in eral regional subspecies (Padilla and Dowler, 1965; modern tapirs. Here, we present a quantitative assess- ment of tapir upper forelimb osteology using three- Wilson and Reeder, 2005): the Baird’s tapir (T. dimensional geometric morphometrics to test whether bairdii), lowland tapir (T. terrestris), mountain the four modern tapir species are monomorphic in their tapir (T. pinchaque), and the Malayan tapir (T. forelimb skeleton. The shape of the upper forelimb bones indicus). Extant tapirs primarily inhabit tropical across four species (T. indicus; T. bairdii; T. terrestris; T. rainforest, with some populations also occupying pinchaque) was investigated. Bones were laser scanned wet grassland and chaparral biomes (Padilla and to capture surface morphology and 3D landmark analysis Dowler, 1965; Padilla et al., 1996). was used to quantify shape. -

United States
DEPARTMENT OF THE INTERIOR BULLETIN OF THE UNITED STATES ISTo. 146 WASHINGTON GOVERNMENT Pit IN TING OFFICE 189C UNITED STATES GEOLOGICAL SURVEY CHAKLES D. WALCOTT, DI11ECTOK BIBLIOGRAPHY AND INDEX NORTH AMEEICAN GEOLOGY, PALEONTOLOGY, PETEOLOGT, AND MINERALOGY THE YEA.R 1895 FEED BOUGHTON WEEKS WASHINGTON Cr O V E U N M K N T P K 1 N T I N G OFFICE 1890 CONTENTS. Page. Letter of trail smittal...... ....................... .......................... 7 Introduction.............'................................................... 9 List of publications examined............................................... 11 Classified key to tlio index .......................................... ........ 15 Bibliography ............................................................... 21 Index....................................................................... 89 LETTER OF TRANSMITTAL DEPARTMENT OF THE INTEEIOE, UNITED STATES GEOLOGICAL SURVEY, DIVISION OF GEOLOGY, Washington, D. 0., June 23, 1896. SIR: I have the honor to transmit herewith the manuscript of a Bibliography and Index of North American Geology, Paleontology, Petrology, and Mineralogy for the year 1895, and to request that it be published as a bulletin of the Survey. Very respectfully, F. B. WEEKS. Hon. CHARLES D. WALCOTT, Director United States Geological Survey. 1 BIBLIOGRAPHY AND INDEX OF NORTH AMERICAN GEOLOGY, PALEONTOLOGY, PETROLOGY, AND MINER ALOGY FOR THE YEAR 1895. By FRED BOUGHTON WEEKS. INTRODUCTION. The present work comprises a record of publications on North Ameri can geology, paleontology, petrology, and mineralogy for the year 1895. It is planned on the same lines as the previous bulletins (Nos. 130 and 135), excepting that abstracts appearing in regular periodicals have been omitted in this volume. Bibliography. The bibliography consists of full titles of separate papers, classified by authors, an abbreviated reference to the publica tion in which the paper is printed, and a brief summary of the con tents, each paper being numbered for index reference. -

Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming
Distribution and Stratigraphip Correlation of Upper:UB_ • Ju Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming U,S. GEOLOGICAL SURVEY PROFESS IONAL PAPER 1540 Cover. A member of the American Museum of Natural History 1896 expedition enter ing the badlands of the Willwood Formation on Dorsey Creek, Wyoming, near what is now U.S. Geological Survey fossil vertebrate locality D1691 (Wardel Reservoir quadran gle). View to the southwest. Photograph by Walter Granger, courtesy of the Department of Library Services, American Museum of Natural History, New York, negative no. 35957. DISTRIBUTION AND STRATIGRAPHIC CORRELATION OF UPPER PALEOCENE AND LOWER EOCENE FOSSIL MAMMAL AND PLANT LOCALITIES OF THE FORT UNION, WILLWOOD, AND TATMAN FORMATIONS, SOUTHERN BIGHORN BASIN, WYOMING Upper part of the Will wood Formation on East Ridge, Middle Fork of Fifteenmile Creek, southern Bighorn Basin, Wyoming. The Kirwin intrusive complex of the Absaroka Range is in the background. View to the west. Distribution and Stratigraphic Correlation of Upper Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Tatman Formations, Southern Bighorn Basin, Wyoming By Thomas M. Down, Kenneth D. Rose, Elwyn L. Simons, and Scott L. Wing U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1540 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsch, Acting Director For sale by U.S. Geological Survey, Map Distribution Box 25286, MS 306, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. -

Resolving the Relationships of Paleocene Placental Mammals
Biol. Rev. (2015), pp. 000–000. 1 doi: 10.1111/brv.12242 Resolving the relationships of Paleocene placental mammals Thomas J. D. Halliday1,2,∗, Paul Upchurch1 and Anjali Goswami1,2 1Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, U.K. 2Department of Genetics, Evolution and Environment, University College London, Gower Street, London WC1E 6BT, U.K. ABSTRACT The ‘Age of Mammals’ began in the Paleocene epoch, the 10 million year interval immediately following the Cretaceous–Palaeogene mass extinction. The apparently rapid shift in mammalian ecomorphs from small, largely insectivorous forms to many small-to-large-bodied, diverse taxa has driven a hypothesis that the end-Cretaceous heralded an adaptive radiation in placental mammal evolution. However, the affinities of most Paleocene mammals have remained unresolved, despite significant advances in understanding the relationships of the extant orders, hindering efforts to reconstruct robustly the origin and early evolution of placental mammals. Here we present the largest cladistic analysis of Paleocene placentals to date, from a data matrix including 177 taxa (130 of which are Palaeogene) and 680 morphological characters. We improve the resolution of the relationships of several enigmatic Paleocene clades, including families of ‘condylarths’. Protungulatum is resolved as a stem eutherian, meaning that no crown-placental mammal unambiguously pre-dates the Cretaceous–Palaeogene boundary. Our results support an Atlantogenata–Boreoeutheria split at the root of crown Placentalia, the presence of phenacodontids as closest relatives of Perissodactyla, the validity of Euungulata, and the placement of Arctocyonidae close to Carnivora. Periptychidae and Pantodonta are resolved as sister taxa, Leptictida and Cimolestidae are found to be stem eutherians, and Hyopsodontidae is highly polyphyletic. -

Late Paleocene) of the Eastern Crazy Mountain Basin, Montana
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. 26, NO. 9, p. 157-196 December 3 1, 1983 MAMMALIAN FAUNA FROM DOUGLASS QUARRY, EARLIEST TIFFANIAN (LATE PALEOCENE) OF THE EASTERN CRAZY MOUNTAIN BASIN, MONTANA BY DAVID W. KRAUSE AND PHILIP D. GINGERICH MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Philip D. Gingerich, Director Gerald R. Smith, Editor This series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan, 48 109. VOLS. 11-XXVI. Parts of volumes may be obtained if available. Price lists available upon inquiry. MAMMALIAN FAUNA FROM DOUGLASS QUARRY, EARLIEST TIFFANIAN (LATE PALEOCENE) OF THE EASTERN CRAZY MOUNTAIN BASIN, MONTANA BY David W. ~rause'and Philip D. ~in~erich' Abstract.-Douglass Quarry is the fourth major locality to yield fossil mammals in the eastern Crazy Mountain Basin of south-central Montana. It is stratigraphically intermediate between Gidley and Silberling quarries below, which are late Torrejonian (middle Paleocene) in age, and Scarritt Quarry above, which is early Tiffanian (late Paleocene) in age. The stratigraphic position of Douglass Quarry and the presence of primitive species of Plesiadapis, Nannodectes, Phenacodus, and Ectocion (genera first appearing at the Torrejonian-Tiffanian boundary) combine to indicate an earliest Tiffanian age. -

Paleontological Discoveries in the Chorrillo Formation (Upper Campanian-Lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina
Rev. Mus. Argentino Cienc. Nat., n.s. 21(2): 217-293, 2019 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) Paleontological discoveries in the Chorrillo Formation (upper Campanian-lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina Fernando. E. NOVAS1,2, Federico. L. AGNOLIN1,2,3, Sebastián ROZADILLA1,2, Alexis M. ARANCIAGA-ROLANDO1,2, Federico BRISSON-EGLI1,2, Matias J. MOTTA1,2, Mauricio CERRONI1,2, Martín D. EZCURRA2,5, Agustín G. MARTINELLI2,5, Julia S. D´ANGELO1,2, Gerardo ALVAREZ-HERRERA1, Adriel R. GENTIL1,2, Sergio BOGAN3, Nicolás R. CHIMENTO1,2, Jordi A. GARCÍA-MARSÀ1,2, Gastón LO COCO1,2, Sergio E. MIQUEL2,4, Fátima F. BRITO4, Ezequiel I. VERA2,6, 7, Valeria S. PEREZ LOINAZE2,6 , Mariela S. FERNÁNDEZ8 & Leonardo SALGADO2,9 1 Laboratorio de Anatomía Comparada y Evolución de los Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina - fernovas@yahoo. com.ar. 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. 3 Fundación de Historia Natural “Felix de Azara”, Universidad Maimonides, Hidalgo 775, C1405BDB Buenos Aires, Argentina. 4 Laboratorio de Malacología terrestre. División Invertebrados Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 5 Sección Paleontología de Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 6 División Paleobotánica. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 7 Área de Paleontología. Departamento de Geología, Universidad de Buenos Aires, Pabellón 2, Ciudad Universitaria (C1428EGA) Buenos Aires, Argentina. 8 Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-INIBIOMA), Quintral 1250, 8400 San Carlos de Bariloche, Río Negro, Argentina. -
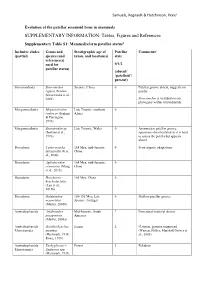
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -
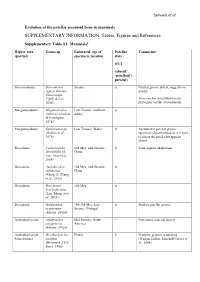
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

To Link and Cite This Article: Doi
Submitted: October 1st, 2019 – Accepted: June 8th, 2020 – Published online: June 11th, 2020 To link and cite this article: doi: https://doi.org/10.5710/AMGH.08.06.2020.3306 1 COTYLAR FOSSA, ITS INTERPRETATION AND FUNCTIONALITY. 2 THE CASE FROM SOUTH AMERICAN NATIVE UNGULATES 3 4 MALENA LORENTE1,2, 3 5 1 División Paleontología de Vertebrados, Museo de La Plata. Paseo del Bosque s/n B1900FWA, La 6 Plata, Buenos Aires, Argentina; [email protected]; 2 CONICET; 3 Facultad de Ciencias 7 Naturales y Museo 8 9 Number of pages: 21, 7 figures 10 Proposed header: LORENTE, COTYLAR FOSSA 1 11 12 Abstract. The cotylar fossa is a complex anatomical character in the astragalar medial malleolar 13 facet. It represents a dynamic relationship between the astragalus and the tibia in the upper tarsal 14 joint. The astragalus must accommodate the medial tibial malleolus when the tibia is in an extreme 15 flexion. It is one of the three morphological synapomorphies considered for Afrotheria, although it 16 is also a recurrent trait among different groups of mammals. Here, fossil South American Native 17 Ungulates and extant mammals were surveyed to reevaluate how much this character is spread and 18 how variable it is. Beyond afrotherians, it is observed that it also appears in primates, macropodid 19 marsupials, laurasiatherian archaic ungulates, perissodactyls, pantodonts, and dinoceratans; it is also 20 in some but not all of the extinct endemic ungulates from South America. No function has been 21 suggested before for the presence of a cotylar fossa. The cotylar fossa could be an adaptation to a 22 passive, rest related posture. -

Bericht Des Naturwissenschaftlichen Vereins Für Schwaben
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.biologiezentrum.at Yer^eichiiiss der bisher bekannten fossilen Säugethiere. Neu zusammengestellt I>i*. Otto I^og-er, kgl. Regierungs- und Kreis-Mediziualratli in Augsburg. 1896. © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.biologiezentrum.at — © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.biologiezentrum.at A. Untsrkksss. Epkcsntalia. 1. Ordnung. Monotremata, Kloakenthiere. (Oniithodelphia, Blainv. Prototheria, Gill.) Echidna gigantea, Krefft. Pleistocän von Australien. — Krefft, Ann. Mag. Nat. Hist. 1868. pag. 113. — Owen, Phil. Trans. Roy. Soc. London. V. 175. 1885. pag. 273. PI. XIV. — Syn. : Ech. Rarasayi, Owen. — Proecliidna Oweni, Krefft. — • Di deilotherium Ye]ierandum,Am. Tertiär von Patagonien . Amegbino, Contrib. Conoc. Mamif. fos. Rep. Argcnt. 1889 pag. 656. 920. PI. XL. Fig. 22. — Amegbino, Enum. syno^^t. Mamif. foss. Patag. 1894. pag. 182. — Syn.: Deilotberium ven., Am. Scotaeops simplex, Am. Tertiär von Patagonien. — Ame- gbino, 1. c. 1889. pag. 658. — Amegbino, 1. c. 1894. pag. 183. Adiastaltus babilis, Am. Tertiär von Patagonien. — A m e- gbino, 1. c. 1891. pag. 184. Ad. procerus. Am. Tertiär von Patagonien. — Amegbino. 1. c. 1894. pag. 186. Plagiocoelus obliquus. Am. Tertiär von Patagonien. — Amegbino, 1. c. 1894. pag. 186. Anatbitus revelator. Am. Tertiär von Patagonien. — Amegbino, 1. c. 1894. pag. 186. 2. Ordnung. Marsupialia, Beutelthiere. (Didelpbia Blv., Metatberia Huxley.) 1. Unterordnung^ Allotheria, Marsh. (Multituberculata, Cope.) Owen. Monogr. Foss. Maram. Mesoz. Form. — Paläontol. Soc. Vol. XXIV. 1871. Marsb, Jurassic Mamni. — Amer. Journ. Sc. XV. 1878. — XVIIL 1879. — XX. 1880. — XXI. 1881. Marsb, Discov. Cretac. Mamm. — Am. Journ. Sc. XXXVIII. 1889. — XLIIl. 1892. Osborn, Struct. and Classif. Mesoz.