Insect Conservation a Handbook of Approaches and Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notts LWS Handbook Part 2A
By NCC PLACE at 12:06 pm, Nov 21, 2017 Ecological Impact Assessment Proposed Eastern Extension to Bestwood Quarry II, Papplewick in Nottinghamshire. Produced by Crestwood Environmental Ltd. Document Reference: CE-BS-1034-RP07 - Final 10 November 2017 Ecological Impact Assessment Proposed Eastern Extension - Bestwood Quarry II CONTENTS 1 INTRODUCTION ........................................................................................................................ 3 1.1 PREAMBLE ..................................................................................................................... 3 1.2 REPORT STRUCTURE ...................................................................................................... 4 1.3 TERMINOLOGY ............................................................................................................... 5 2 METHODOLOGY AND METHODS OF ASSESSMENT ..................................................................... 5 2.1 SCOPE OF THE ASSESSMENT.......................................................................................... 5 2.2 BASELINE CONDITIONS .................................................................................................. 6 2.3 DESK STUDY ................................................................................................................... 6 2.4 FIELD SURVEYS ............................................................................................................... 7 2.5 SCOPING OPINION CONSULTEE RESPONSES ON ECOLOGICAL MATTERS .................... -

Portsmouth Number List 2019
Portsmouth Number List 2019 The RYA Portsmouth Yardstick Scheme is provided to enable clubs to allow boats of different classes to race against each other fairly. The RYA actively encourages clubs to adjust handicaps where classes are either under or over performing compared to the number being used. The Portsmouth Yardstick list combines the Portsmouth numbers with class configuration and the total number of races returned to the RYA in the annual return. This additional data has been provided to help clubs achieve the stated aims of the Portsmouth Yardstick system and make adjustments to Portsmouth Numbers where necessary. Clubs using the PN list should be aware that the list is based on the typical performance of each boat across a variety of clubs and locations. Experimental numbers are based on fewer returns and are to be used as a guide for clubs to allocate as a starting number before reviewing and adjusting where necessary. The list of experimental Portsmouth Numbers will be periodically reviewed by the RYA and is based on data received via PY Online. Users of the PY scheme are reminded that all Portsmouth Numbers published by the RYA should be regarded as a guide only. The RYA list is not definitive and clubs should adjust where necessary. For further information please visit the RYA website: http://www.rya.org.uk/racing/Pages/portsmouthyardstick.aspx RYA PN LIST - Dinghy No. of Change Class Name Rig Spinnaker Number Races Notes Crew from '18 420 2 S C 1111 0 428 2000 2 S A 1112 3 2242 29ER 2 S A 907 -5 277 505 2 S C 903 0 277 -

IT's a WINNER! Refl Ecting All That's Great About British Dinghy Sailing
ALeXAnDRA PALACe, LOnDOn 3-4 March 2012 IT'S A WINNER! Refl ecting all that's great about British dinghy sailing 1647 DS Guide (52).indd 1 24/01/2012 11:45 Y&Y AD_20_01-12_PDF.pdf 23/1/12 10:50:21 C M Y CM MY CY CMY K The latest evolution in Sailing Hikepant Technology. Silicon Liquid Seam: strongest, lightest & most flexible seams. D3O Technology: highest performance shock absorption, impact protection solutions. Untitled-12 1 23/01/2012 11:28 CONTENTS SHOW ATTRACTIONS 04 Talks, seminars, plus how to get to the show and where to eat – all you need to make the most out of your visit AN OLYMPICS AT HOME 10 Andy Rice speaks to Stephen ‘Sparky’ Parks about the plus and minus points for Britain's sailing team as they prepare for an Olympic Games on home waters SAIL FOR GOLD 17 How your club can get involved in celebrating the 2012 Olympics SHOW SHOPPING 19 A range of the kit and equipment on display photo: rya* photo: CLubS 23 Whether you are looking for your first club, are moving to another part of the country, or looking for a championship venue, there are plenty to choose WELCOME SHOW MAP enjoy what’s great about British dinghy sailing 26 Floor plans plus an A-Z of exhibitors at the 2012 RYA Volvo Dinghy Show SCHOOLS he RYA Volvo Dinghy Show The show features a host of exhibitors from 29 Places to learn, or improve returns for another year to the the latest hi-tech dinghies for the fast and your skills historical Alexandra Palace furious to the more traditional (and stable!) in London. -

UC Merced Frontiers of Biogeography
UC Merced Frontiers of Biogeography Title Bucking the trend: the diversity of Anthropocene ‘winners’ among British moths Permalink https://escholarship.org/uc/item/6hz0x33v Journal Frontiers of Biogeography, 11(3) Authors Boyes, Douglas H. Fox, Richard Shortall, Chris R. et al. Publication Date 2019 DOI 10.21425/F5FBG43862 Supplemental Material https://escholarship.org/uc/item/6hz0x33v#supplemental License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California a Frontiers of Biogeography 2019, 11.3, e43862 Frontiers of Biogeography RESEARCH ARTICLE the scientific journal of the International Biogeography Society Bucking the trend: the diversity of Anthropocene ‘winners’ among British moths Douglas H. Boyes1,*, Richard Fox2, Chris R. Shortall3 and Robert J. Whittaker1,4 1 School of Geography and the Environment, University of Oxford, South Parks Road, Oxford, OX1 3QY, UK; 2 Butterfly Conservation, Manor Yard, East Lulworth, Dorset, BH20 5QP, UK; 3 Rothamsted Insect Survey, Rothamsted Research, Harpenden AL5 2JQ, UK and 4 Center for Macroecology, Evolution and Climate, GLOBE Institute, University of Copenhagen, Copenhagen, Denmark. *Correspondence: Douglas H. Boyes, Centre for Ecology & Hydrology, Wallingford, OX10 8BB, UK. E-mail: [email protected]. Abstract Highlights An appreciation of how some species are becoming more • Trends in abundance and occurrence are described common despite unprecedented anthropogenic pressures for 51 British macro-moths that have become more could offer key insights for mitigating the global biodiversity common, using data from two long-term recording crisis. Research to date has largely focused on declining schemes species, while species that are becoming more common have received relatively little attention. -

A Proteomic Approach for Studying Insect Phylogeny: CAPA Peptides of Ancient Insect Taxa (Dictyoptera, Blattoptera) As a Test Case
BMC Evolutionary Biology BioMed Central Research article Open Access A proteomic approach for studying insect phylogeny: CAPA peptides of ancient insect taxa (Dictyoptera, Blattoptera) as a test case Steffen Roth1,3, Bastian Fromm1, Gerd Gäde2 and Reinhard Predel*1 Address: 1Institute of Zoology, University of Jena, Erbertstrasse 1, D-07743 Jena, Germany, 2Zoology Department, University of Cape Town, Rondebosch 7701, South Africa and 3Institute of Biology, University of Bergen, Bergen N-5020, Norway Email: Steffen Roth - [email protected]; Bastian Fromm - [email protected]; Gerd Gäde - [email protected]; Reinhard Predel* - [email protected] * Corresponding author Published: 3 March 2009 Received: 6 October 2008 Accepted: 3 March 2009 BMC Evolutionary Biology 2009, 9:50 doi:10.1186/1471-2148-9-50 This article is available from: http://www.biomedcentral.com/1471-2148/9/50 © 2009 Roth et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Neuropeptide ligands have to fit exactly into their respective receptors and thus the evolution of the coding regions of their genes is constrained and may be strongly conserved. As such, they may be suitable for the reconstruction of phylogenetic relationships within higher taxa. CAPA peptides of major lineages of cockroaches (Blaberidae, Blattellidae, Blattidae, Polyphagidae, Cryptocercidae) and of the termite Mastotermes darwiniensis were chosen to test the above hypothesis. The phylogenetic relationships within various groups of the taxon Dictyoptera (praying mantids, termites and cockroaches) are still highly disputed. -
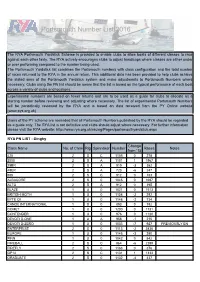
Portsmouth Number List 2016
Portsmouth Number List 2016 The RYA Portsmouth Yardstick Scheme is provided to enable clubs to allow boats of different classes to race against each other fairly. The RYA actively encourages clubs to adjust handicaps where classes are either under or over performing compared to the number being used. The Portsmouth Yardstick list combines the Portsmouth numbers with class configuration and the total number of races returned to the RYA in the annual return. This additional data has been provided to help clubs achieve the stated aims of the Portsmouth Yardstick system and make adjustments to Portsmouth Numbers where necessary. Clubs using the PN list should be aware that the list is based on the typical performance of each boat across a variety of clubs and locations. Experimental numbers are based on fewer returns and are to be used as a guide for clubs to allocate as a starting number before reviewing and adjusting where necessary. The list of experimental Portsmouth Numbers will be periodically reviewed by the RYA and is based on data received from the PY Online website (www.pys.org.uk). Users of the PY scheme are reminded that all Portsmouth Numbers published by the RYA should be regarded as a guide only. The RYA list is not definitive and clubs should adjust where necessary. For further information please visit the RYA website: http://www.rya.org.uk/racing/Pages/portsmouthyardstick.aspx RYA PN LIST - Dinghy Change Class Name No. of Crew Rig Spinnaker Number Races Notes from '15 420 2 S C 1105 0 278 2000 2 S A 1101 1 1967 29ER 2 S A -

Phylogeny and Life History Evolution of Blaberoidea (Blattodea)
78 (1): 29 – 67 2020 © Senckenberg Gesellschaft für Naturforschung, 2020. Phylogeny and life history evolution of Blaberoidea (Blattodea) Marie Djernæs *, 1, 2, Zuzana K otyková Varadínov á 3, 4, Michael K otyk 3, Ute Eulitz 5, Kla us-Dieter Klass 5 1 Department of Life Sciences, Natural History Museum, London SW7 5BD, United Kingdom — 2 Natural History Museum Aarhus, Wilhelm Meyers Allé 10, 8000 Aarhus C, Denmark; Marie Djernæs * [[email protected]] — 3 Department of Zoology, Faculty of Sci- ence, Charles University, Prague, 12844, Czech Republic; Zuzana Kotyková Varadínová [[email protected]]; Michael Kotyk [[email protected]] — 4 Department of Zoology, National Museum, Prague, 11579, Czech Republic — 5 Senckenberg Natural History Collections Dresden, Königsbrücker Landstrasse 159, 01109 Dresden, Germany; Klaus-Dieter Klass [[email protected]] — * Corresponding author Accepted on February 19, 2020. Published online at www.senckenberg.de/arthropod-systematics on May 26, 2020. Editor in charge: Gavin Svenson Abstract. Blaberoidea, comprised of Ectobiidae and Blaberidae, is the most speciose cockroach clade and exhibits immense variation in life history strategies. We analysed the phylogeny of Blaberoidea using four mitochondrial and three nuclear genes from 99 blaberoid taxa. Blaberoidea (excl. Anaplectidae) and Blaberidae were recovered as monophyletic, but Ectobiidae was not; Attaphilinae is deeply subordinate in Blattellinae and herein abandoned. Our results, together with those from other recent phylogenetic studies, show that the structuring of Blaberoidea in Blaberidae, Pseudophyllodromiidae stat. rev., Ectobiidae stat. rev., Blattellidae stat. rev., and Nyctiboridae stat. rev. (with “ectobiid” subfamilies raised to family rank) represents a sound basis for further development of Blaberoidea systematics. -

Portsmouth Yardstick 2010 2
Standard Portmouth Yardstick Numbers Complete Numerical Listing Valid from 9th March 2010. Class PY NO Pursuit Race No Class PY NO Pursuit Race No 18' Skiff a 675 68 Seafly c 1087 109 Foiling Moth 690 69 420 c 1087 109 49er a 744 74 405 a 1089 109 RS 800 a 822 82 Laser 2000 a 1090 109 International 14 * Non Foil 840 84 National 12 * o 1089 109 Laser 5000 a 846 85 Redwing c 1094 109 Boss a 847 85 Wayfarer c 1101 110 RS 700 a 857 86 Laser Vago SD S/H a 1100 110 Canoe International a 870 87 Laser Radial o 1104 110 B 14 a 874 87 OK o 1109 111 Musto Skiff a 875 88 Leader c 1115 112 Flying Dutchman c 879 88 Enterprise o 1116 112 505 c 902 90 Snipe o 1117 112 RS K6 a 903 90 GP 14 c 1127 113 Canoe International o 905 91 Wanderer c 1139 114 59er a 905 91 GP 14 o 1136 114 Laser 4000 a 908 91 Europe o 1140 114 RS 600 o 920 92 Byte CII o 1140 114 29er a 924 92 Lightening 368 o 1152 115 ISO a 926 93 Mercury c 1152 115 Javelin c 926 93 Solo o 1155 116 Cherub * 2005 a 930 93 Byte o 1165 117 Spice a 930 93 Firefly o 1165 117 Laser Vortex a 937 94 Streaker o 1162 116 Osprey c 940 94 Graduate o 1165 117 RS 400 a 950 95 British Moth o 1168 117 National 18 c 957 96 Comet o 1177 118 Laser Vortex o 960 96 Comet Duo o 1175 118 RS 500 a 972 97 Laser 4.7 o 1175 118 470 c 973 97 Miracle c 1185 119 Hornet c 973 97 Splash o 1184 118 Cherub * 1997 a 975 98 RS Feva XL a 1200 120 International Moth * Non Foil 980 98 Topaz Duo o 1190 119 Fireball c 982 98 Pacer o 1193 119 Contender o 993 99 Bosun c 1198 120 RS 300 o 1000 100 Challenger o 1200 120 Buzz a 1002 100 YW -

Diversity and Abundance of Insects at the Campus of Higher Polytechnic Institute of Manica, Mozambique
Asian Journal of Research in Zoology 4(1): 34-39, 2021; Article no.AJRIZ.60374 ISSN: 2582-466X Diversity and Abundance of Insects at the Campus of Higher Polytechnic Institute of Manica, Mozambique António C. Manhice1,2* 1Departament of Economy, Management and Tourism, EN6, Vanduzi, 417, Manica, Mozambique. 2Laboratory of Biodiversity, EN6, Vanduzi, 417, Manica, Mozambique. Author’s contribution The sole author designed, analysed, interpreted and prepared the manuscript. Article Information DOI: 10.9734/AJRIZ/2021/v4i130106 Editor(s): (1) Dr. Farzana Khan Perveen, Shaheed Benazir Bhutto University, Pakistan. Reviewers: (1) R. Velusamy, Tamil Nadu Veterinary and Animal Sciences University, India. (2) S. B. Das, Jawaharlal Nehru Agricultural University, India. (3) Augusto Henrique Batista Rosa, University of Campinas, Brazil. Complete Peer review History: http://www.sdiarticle4.com/review-history/60374 Received 30 June 2020 Original Research Article Accepted 08 September 2020 Published 19 March 2021 ABSTRACT The aim of this study was to assess insect diversity and abundance at the Campus of Higher Polytechnic Institute of Manica, Mozambique. Collection of insects was carried out by pitfall traps a long linear transects and sweep nets during 21 days of October 2019. Data were analyzed using descriptive statistical and Shannon diversity index. A total of 1780 individuals of insects belonging to 8 orders, 15 families and 27 species were collected. Highest relative abundance was observed in Hymenoptera order (92.64%), and the least were Blattodea (2.70%), Diptera (2.13%), Coleoptera (0.82%), Orthoptera (1.52%), Phasmatodea (0.06%), Mantodea (0.28%) and Hemiptera (0.11%). The abundant specie was Crematogaster peringueyi (Hymenoptera) with 89.83% follwed by Macrotermes natalensis (Blattodea) and Chrysonmya chloropyga (Diptera) with 2.47% and 1.63% respectively. -
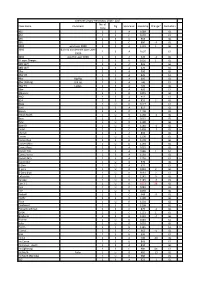
Class Name Comment No. of Crew Rig Spinnaker Handicap Change
Grafham Dinghy Handicaps, 2016 - 2017 No. of Class Name Comment Rig Spinnaker Handicap Change* Derivation Crew 405 2 S A 1089 GL 420 2 S C 1070 GL 470 2 S C 963 GL 505 2 S C 880 -3 GL 2000 was Laser 2000 2 S A 1101 1 GL 3000 Built by Vandercraft (was Laser 2 S A 1007 GL 3000) 4000 was the Laser 4000 2 S A 920 -2 GL 12 sqm Sharpie 2 S 0 1026 1 GL 12ft Skiff 3 S A 835 GL 18ft Skiff 2 S A 670 GL x 29er 2 S A 900 GL 29er XX 2 S A 820 GL 49er Big Rig 2 S A 695 GL 49er (Old rig) Old rig 2 S A 740 GL 49er FX Ladies 2 S A 720 GL 59er 2 S A 905 GL Albacore 2 S 0 1055 GL AltO 2 S A 912 GL B14 2 S A 852 2 GL Blaze 1 U 0 1027 GL Boss 2 S A 817 GL Bosun 2 S 0 1198 GL British Moth 1 U 0 1158 -2 GL Buzz 2 S A 1015 GL Byte 1 U 0 1190 GL Byte CII 1 U 0 1148 -2 GL Cadet 2 S C 1428 -7 GL x Cherub 2 S A 890 GL Comet 1 U 0 1200 GL Comet Duo 2 S 0 1178 GL Comet Mino 1 U 0 1193 GL Comet Race 2 S A 1025 GL Comet Trio 2 S A 1086 1 GL Comet Versa 2 S A 1150 GL Comet Zero 2 S A 1250 GL x Contender 1 U 0 976 GL D-One 1 U A 971 GL D-Zero 1 U 0 1033 2 GL D-Zero Blue 1 U 0 1061 2 GL Enterprise 2 S 0 1145 3 GL Europe 1 U 0 1145 -3 GL Farr 3.7 1 U 0 1053 38 GL Finn 1 U 0 1042 GL Fire 1 U 0 1050 GL Fireball 2 S C 948 -5 GL Firefly 2 S 0 1190 GL Flash 1 U 0 1101 GL Fleetwind 2 S 0 1268 GL x Flying Dutchman 2 S C 879 -1 GL GP14 2 S C 1131 1 GL Graduate 2 S 0 1132 -4 GL Hadron 1 U 0 1040 GL Halo 1 U 0 1010 GL Heron 1 S 0 1345 GL x Hornet 2 S C 962 -1 GL Icon 2 S 0 990 GL Int 14 2 S A 700 -20 GL Int Canoe 1 S 0 893 GL Int Canoe - Asym 1 S A 840 GL Int Lightning 3 S C 980 -

The Life of William Prest, York Entomologist (1824-1884)
This is a repository copy of The Life of William Prest, York Entomologist (1824-1884). White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/166385/ Version: Published Version Article: Mayhew, Peter John orcid.org/0000-0002-7346-6560 (Accepted: 2020) The Life of William Prest, York Entomologist (1824-1884). The Naturalist. pp. 132-151. (In Press) Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The Life of William Prest, York Entomologist (1824-1884) Peter J. Mayhew Department of Biology, University of York, Heslington, York YO10 5DD Email: [email protected] William Prest was one of the most influential Nineteenth Century entomologists from the York area. He was a skilled and energetic collector of Lepidoptera and an enthusiastic communicator and compiler of natural history information. At different times in his life he organized two local natural history societies from his own home: the York Entomological Society (YES) and the York and District Field Naturalists’ Society (YDFNS), the latter lasting over a hundred years. -

The Mirror Book MIRROR SAILING from START to FINISH
The Mirror Book Second edition by Peter Aitken & Tim Davison Published by Fernhurst Books; 11th September 2017; RRP: £16.99 MIRROR SAILING FROM START TO FINISH The Mirror dinghy was a significant factor in the boom of dinghy sailing in the 1960s, introducing thousands to the delights of sailing. While sailing has come a long way since then and there have been a lot of far more exotic craft launched, the Mirror remains a fantastic boat in which to learn to sail and to compete at the highest levels. As befits such an iconic dinghy, Fernhurst Books is proud to publish the second edition of The Mirror Book, bringing it into the Start to Finish format alongside such revered books as The Laser Book, The Topper Book, The Catamaran Book and The Foiling Dinghy Book. Like each of these titles, this book tells you everything you need to know about sailing the boat (whether it be the original gaff-rigged dinghy or the newer Bermuda rig). Written for both those starting out through to those striving to win a championship, the book is packed with practical advice and illustrated with step-by-step photographs. It covers: setting up the Mirror and early sailing experiences; developing skills on all points of sailing and with the spinnaker; racing & masterclasses to become a championship winner. Over 180 photographs show you exactly how it’s done, and 72 diagrams illustrate key boat parts, the different points of sailing and complex on-the-water scenarios with clarity. It is written by Peter Aitken, formerly the UK National Mirror Coach, who has coached teams to win world championships in the Mirror, Cadet and 420 classes, and Tim Davison, a successful racer and author of countless books on sailing.