The Anti-Apoptotic Role of Neurotensin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcitonin Gene Products and the Kidney
Kiinische Klin Wochenschr (1989) 67:870-875 W°chenchrif t © Springer-Verlag 1989 Calcitonin Gene Products and the Kidney A. Kurtz 1, R. Muff z, and J.A. Fischer z 1 Physiologic Institute, University of Ziirich, Switzerland 2 Research Laboratory for Calcium Metabolism, Departments of Orthopedic Surgery and Medicine, University of Ziirich, Ziirich, Switzerland Summary. Calcitonin gene-related peptide (CGRP) Calcitonin and CGRP are single chain polypep- is localized in capsaicin-sensitive nerve fibres in the tides consisting of 32 and 37 amino acids, respec- kidney and urogenital tract whereas calcitonin tively. They have in common amino-terminal ring reaches the kidney through the general circulation. structures linked by disulfide bridges and the car- Systemic infusion of CGRP and perfusion of iso- boxyltermini are amidated. In man, CGRP shares lated rat kidney reduces vascular resistance, and 16% structural homology with calcitonin whereas increases renal blood flow and glomerular filtra- the homology between CGRP-I and -II is 92% tion. CGRP stimulates renin secretion in vivo and [13]. As a result, distinct receptors for calcitonin in vitro and inhibits contraction of isolated rat me- and CGRP have been identified [7, 11, 33, 42]. sangial cells by angiotensin II. Calcitonin does not Human CGRP-I and -II, due to their high homolo- affect vascular resistance, renal blood flow and glo- gy, crossreact almost completely, but subtle differ- merular filtration, and is tess potent in stimulating ences in the distribution of human CGRP-I and renin secretion, and does not alter contraction of -II binding sites have been observed on receptor isolated rat mesangial cells by angiotensin II. -

From Inverse Agonism to 'Paradoxical Pharmacology' Richard A
International Congress Series 1249 (2003) 27-37 From inverse agonism to 'Paradoxical Pharmacology' Richard A. Bond*, Kenda L.J. Evans, Zsirzsanna Callaerts-Vegh Department of Pharmacological and Pharmaceutical Sciences, University of Houston, 521 Science and Research Bldg 2, 4800 Caltioun, Houston, TX 77204-5037, USA Received 16 April 2003; accepted 16 April 2003 Abstract The constitutive or spontaneous activity of G protein-coupled receptors (GPCRs) and compounds acting as inverse agonists is a recent but well-established phenomenon. Dozens of receptor subtypes for numerous neurotransmitters and hormones have been shown to posses this property. However, do to the apparently low percentage of receptors in the spontaneously active state, the physiologic relevance of these findings remains questionable. The possibility that the reciprocal nature of the effects of agonists and inverse agonists may extend to cellular signaling is discussed, and that this may account for the beneficial effects of certain p-adrenoceptor inverse agonists in the treatment of heart failure. © 2003 Elsevier Science B.V. All rights reserved. Keywords. Inverse agonism; GPCR; Paradoxical pharmacology 1. Brief history of inverse agonism at G protein-coupled receptors For approximately three-quarters of a century, ligands that interacted with G protein- coupled receptors (GPCRs) were classified either as agonists or antagonists. Receptors were thought to exist in a single quiescent state that could only induce cellular signaling upon agonist binding to the receptor to produce an activated state of the receptor. In this model, antagonists had no cellular signaling ability on their own, but did bind to the receptor and prevented agonists from being able to bind and activate the receptor. -
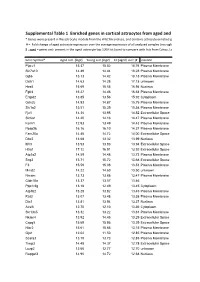
Supplemental Table 1 Enriched Genes in Cortical Astrocytes from Aged
Supplemental Table 1 Enriched genes in cortical astrocytes from aged and young-adult mice * Genes were present in the astrocyte module from the WGCNA analysis, and contains astrocyte enriched genes compared to microglia and oligodendrocytes # = Fold change of aged astrocyte expression over the average expression of all analyzed samples (microglia, astrocytes: young, old, with and without myelin contamination) $ ; aged = genes only present in the aged astrocyte top 1000 list (used to compare with lists from Cahoy, Lovatt, Doyle; see Fig. 4B), all = genes present in all astrocyte top 1000 lists Gene Symbol* Aged astr. (log2) Young astr.(log2) FC (aged/ aver.)# Location Ptprz1 15.37 15.02 18.76 Plasma Membrane Slc7a10 14.49 14.44 18.28 Plasma Membrane Gjb6 15.13 14.42 18.18 Plasma Membrane Dclk1 14.63 14.28 17.18 unknown Hes5 15.69 15.55 16.94 Nucleus Fgfr3 15.27 14.46 16.54 Plasma Membrane Entpd2 13.85 13.56 15.92 Cytoplasm Grin2c 14.93 14.87 15.75 Plasma Membrane Slc1a2 15.51 15.39 15.58 Plasma Membrane Fjx1 14.36 13.98 14.52 Extracellular Space Slc6a1 14.20 14.16 14.47 Plasma Membrane Kcnk1 12.93 13.49 14.43 Plasma Membrane Ppap2b 16.16 16.10 14.37 Plasma Membrane Fam20a 14.48 14.72 14.00 Extracellular Space Dbx2 13.68 13.32 13.99 Nucleus Itih3 13.93 13.93 13.94 Extracellular Space Htra1 17.12 16.91 13.92 Extracellular Space Atp1a2 14.59 14.48 13.73 Plasma Membrane Scg3 15.71 15.72 13.68 Extracellular Space F3 15.59 15.08 13.51 Plasma Membrane Mmd2 14.22 14.60 13.50 unknown Nrcam 13.73 13.88 13.47 Plasma Membrane Cldn10a 13.37 13.57 13.46 -

Neurotensin Activates Gabaergic Interneurons in the Prefrontal Cortex
The Journal of Neuroscience, February 16, 2005 • 25(7):1629–1636 • 1629 Behavioral/Systems/Cognitive Neurotensin Activates GABAergic Interneurons in the Prefrontal Cortex Kimberly A. Petrie,1 Dennis Schmidt,1 Michael Bubser,1 Jim Fadel,1 Robert E. Carraway,2 and Ariel Y. Deutch1 1Departments of Pharmacology and Psychiatry, Vanderbilt University Medical Center, Nashville, Tennessee 37212, and 2Department of Physiology, University of Massachusetts Medical Center, Worcester, Massachusetts 01655 Converging data suggest a dysfunction of prefrontal cortical GABAergic interneurons in schizophrenia. Morphological and physiological studies indicate that cortical GABA cells are modulated by a variety of afferents. The peptide transmitter neurotensin may be one such modulator of interneurons. In the rat prefrontal cortex (PFC), neurotensin is exclusively localized to dopamine axons and has been suggested to be decreased in schizophrenia. However, the effects of neurotensin on cortical interneurons are poorly understood. We used in vivo microdialysis in freely moving rats to assess whether neurotensin regulates PFC GABAergic interneurons. Intra-PFC administra- tion of neurotensin concentration-dependently increased extracellular GABA levels; this effect was impulse dependent, being blocked by treatment with tetrodotoxin. The ability of neurotensin to increase GABA levels in the PFC was also blocked by pretreatment with 2-[1-(7-chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)pyrazole-3-yl)carbonylamino]tricyclo(3.3.1.1.3.7)decan-2-carboxylic acid (SR48692), a high-affinity neurotensin receptor 1 (NTR1) antagonist. This finding is consistent with our observation that NTR1 was localized to GABAergic interneurons in the PFC, particularly parvalbumin-containing interneurons. Because neurotensin is exclusively localized to dopamine axons in the PFC, we also determined whether neurotensin plays a role in the ability of dopamine agonists to increase extracellular GABA levels. -

Purification of a Galanin Receptor from Pig Brain
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 3845-3849, May 1993 Neurobiology Purification of a galanin receptor from pig brain YAOHUI CHEN*, ALAIN FOURNIERt, ALAIN COUVINEAU*, MARC LABURTHE*, AND BRIGITTE AMIRANOFF*t *Laboratoire de Biologie and Physiologie des Cellules Digestives, Institut National de la Sant6 et de la Recherche Mddicale, U 239, 16 Rue Henri Huchard-75018 Paris, France; and tUniversitd du Quebec, Institut National de la Recherche Scientifique, INRS-Santd, 245 Boulevard Hymus, Pointe Claire, Qudbec, H9R1G6, Canada Communicated by Tomas Hokfelt, January 4, 1993 ABSTRACT A galanin receptor protein was solubilized lished data), we report the purification of a galanin receptor with 3-[(3-cholamidopropyl)dimethylammonio]-1-propane- from pig brain, a rich source of receptors that is available in sulfonate (CHAPS) from pig brain membranes and then pu- large amounts. This represents a basic step toward knowl- rified by single-step affinity chromatography. The product edge of the pharmacology and biochemistry of galanin re- exhibits saturable and specific binding for galanin with a ceptors and should lead to a better understanding of their binding activity of 17 nmol/mg of protein and a dissociation expression in the organism. constant (Kd) of 10 nM. This represents a 300,000-fold puri- fication over the detergent-solubilized fraction with a final recovery of 31% of the initial membrane galanin binding METHODS activity. Gel electrophoresis of the affinity-purified material Materials. Synthetic porcine galanin, glucagon, vasoactive showed a single polypeptide of 54 kDa by silver staining and intestinal peptide, synthetic neurotensin, substance P, baci- after radioiodination. Cross-linking of a purified fraction af- tracin, leupeptin, pepstatin A, GTP, GDP, guanosine 5'-[13,v- rmity-labeled with 125I-labeled galanin revealed a single band imido]triphosphate, cholesteryl hemisuccinate, 3-[(3-cholami- for the galanin-receptor complex at 57 kDa. -

And Neurotensin-Immunoreactive Cells in the Gastrointestinal Tract of the Chicken
Histol Histopath (1989) 4: 55-62 Histology and Histopathology The distribution and ontogeny of gastrin/CCK-, somatostatin- and neurotensin-immunoreactive cells in the gastrointestinal tract of the chicken B.C. Alison Department of Anatomy, Medical School, University of the Witwatersrand,Johannesburg, South Africa Summary. The distribution and time of appearance of wide variety of invertebrates and of vertebrates, cells with gastrin1CCK-, neurotensin- and somatostatin- especially mammals, (Rufener et al., 1975; Sundler et like immunoreactivity were studied in samples from al., 1977; Alumets et al., 1977; Helmstaedter et al., 1977; eight regions of the gastrointestinal tract of chick Seino et al., 1979). Studies on avian gut have been embryos from 11 days of incubation to hatching. No conducted at hatching (Rawdon and Andrew, 1981) and immunoreactive cells were found in any region at 11 thereafter in young birds, (Larsson et al., 1974) and days of incubation. Somatostatin- and neurotensin- adults (Aitken, 1958; Yamada et al., 1979). immunoreactive cells appeared for the first time in the Immunocytochemical studies have also demonstrated proventriculus, pyloric region and duodenum at 12 days immunoreactive cells of various types in early rat and of incubation with cells immunoreactive for neurotensin human foetal gut (Larsson et al., 1975; Dubois et al., occurring in the rectum at the same stage. GastrinICCK- 1976; Dubois et al., 1976; Larsson et al., 1977; Dupouy et immunoreactive cells were detected in the small intestine al., 1983; Kataoka et al., 1985). There is, however, much first at 14 days and in the pyloric region two days later. less published work on embryonic avian material; that of Cells immunoreactive for somatostatin and neurotensin Sundler et al. -

Conformational Dynamics Between Transmembrane Domains And
RESEARCH ARTICLE Conformational dynamics between transmembrane domains and allosteric modulation of a metabotropic glutamate receptor Vanessa A Gutzeit1, Jordana Thibado2, Daniel Starer Stor2, Zhou Zhou3, Scott C Blanchard2,3,4, Olaf S Andersen2,3, Joshua Levitz2,4,5* 1Neuroscience Graduate Program, Weill Cornell Graduate School of Medical Sciences, New York, United States; 2Physiology, Biophysics and Systems Biology Graduate Program, Weill Cornell Graduate School of Medical Sciences, New York, United States; 3Department of Physiology and Biophysics, Weill Cornell Medicine, New York, United States; 4Tri-Institutional PhD Program in Chemical Biology, New York, United States; 5Department of Biochemistry, Weill Cornell Medicine, New York, United States Abstract Metabotropic glutamate receptors (mGluRs) are class C, synaptic G-protein-coupled receptors (GPCRs) that contain large extracellular ligand binding domains (LBDs) and form constitutive dimers. Despite the existence of a detailed picture of inter-LBD conformational dynamics and structural snapshots of both isolated domains and full-length receptors, it remains unclear how mGluR activation proceeds at the level of the transmembrane domains (TMDs) and how TMD-targeting allosteric drugs exert their effects. Here, we use time-resolved functional and conformational assays to dissect the mechanisms by which allosteric drugs activate and modulate mGluR2. Single-molecule subunit counting and inter-TMD fluorescence resonance energy transfer *For correspondence: measurements in living cells -

Guthrie Cdna Resource Center
cDNA Resource Center cDNA Resource Center Catalog cDNA Resource Center Missouri University of Science and Technology 400 W 11th Rolla, MO 65409 TEL: (573) 341-7610 FAX: (573) 341-7609 EMAIL: [email protected] www.cdna.org September, 2008 1 cDNA Resource Center Visit our web site for product updates 2 cDNA Resource Center The cDNA Resource Center The cDNA Resource Center is a service provided by the faculty of the Department of Biological Sciences of Missouri University of Science and Technology. The purpose of the cDNA Resource Center is to further scientific investigation by providing cDNA clones of human proteins involved in signal transduction processes. This is achieved by providing high quality clones for important signaling proteins in a timely manner. By high quality, we mean that the clones are • Sequence verified • Propagated in a versatile vector useful in bacterial and mammalian systems • Free of extraneous 3' and 5' untranslated regions • Expression verified (in most cases) by coupled in vitro transcription/translation assays • Available in wild-type, epitope-tagged and common mutant forms (e.g., constitutively- active or dominant negative) By timely, we mean that the clones are • Usually shipped within a day from when you place your order. Clones can be ordered from our web pages, by FAX or by phone. Within the United States, clones are shipped by overnight courier (FedEx); international orders are shipped International Priority (FedEx). The clones are supplied for research purposes only. Details on use of the material are included on the Material Transfer Agreement (page 3). Clones are distributed by agreement in Invitrogen's pcDNA3.1+ vector. -

Neurotransmitter and Neuropeptide Regulation of Mast Cell Function
Xu et al. Journal of Neuroinflammation (2020) 17:356 https://doi.org/10.1186/s12974-020-02029-3 REVIEW Open Access Neurotransmitter and neuropeptide regulation of mast cell function: a systematic review Huaping Xu1, Xiaoyun Shi2, Xin Li3, Jiexin Zou4, Chunyan Zhou5, Wenfeng Liu5, Huming Shao5, Hongbing Chen5 and Linbo Shi4* Abstract The existence of the neural control of mast cell functions has long been proposed. Mast cells (MCs) are localized in association with the peripheral nervous system (PNS) and the brain, where they are closely aligned, anatomically and functionally, with neurons and neuronal processes throughout the body. They express receptors for and are regulated by various neurotransmitters, neuropeptides, and other neuromodulators. Consequently, modulation provided by these neurotransmitters and neuromodulators allows neural control of MC functions and involvement in the pathogenesis of mast cell–related disease states. Recently, the roles of individual neurotransmitters and neuropeptides in regulating mast cell actions have been investigated extensively. This review offers a systematic review of recent advances in our understanding of the contributions of neurotransmitters and neuropeptides to mast cell activation and the pathological implications of this regulation on mast cell–related disease states, though the full extent to which such control influences health and disease is still unclear, and a complete understanding of the mechanisms underlying the control is lacking. Future validation of animal and in vitro models also is needed, which incorporates the integration of microenvironment-specific influences and the complex, multifaceted cross-talk between mast cells and various neural signals. Moreover, new biological agents directed against neurotransmitter receptors on mast cells that can be used for therapeutic intervention need to be more specific, which will reduce their ability to support inflammatory responses and enhance their potential roles in protecting against mast cell–related pathogenesis. -

Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes
Tohoku J. Exp. Med., 2011,Differential 223, 161-176 Gene Expression in OPCs, Oligodendrocytes and Type II Astrocytes 161 Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes Jian-Guo Hu,1,2,* Yan-Xia Wang,3,* Jian-Sheng Zhou,2 Chang-Jie Chen,4 Feng-Chao Wang,1 Xing-Wu Li1 and He-Zuo Lü1,2 1Department of Clinical Laboratory Science, The First Affiliated Hospital of Bengbu Medical College, Bengbu, P.R. China 2Anhui Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu, P.R. China 3Department of Neurobiology, Shanghai Jiaotong University School of Medicine, Shanghai, P.R. China 4Department of Laboratory Medicine, Bengbu Medical College, Bengbu, P.R. China Oligodendrocyte precursor cells (OPCs) are bipotential progenitor cells that can differentiate into myelin-forming oligodendrocytes or functionally undetermined type II astrocytes. Transplantation of OPCs is an attractive therapy for demyelinating diseases. However, due to their bipotential differentiation potential, the majority of OPCs differentiate into astrocytes at transplanted sites. It is therefore important to understand the molecular mechanisms that regulate the transition from OPCs to oligodendrocytes or astrocytes. In this study, we isolated OPCs from the spinal cords of rat embryos (16 days old) and induced them to differentiate into oligodendrocytes or type II astrocytes in the absence or presence of 10% fetal bovine serum, respectively. RNAs were extracted from each cell population and hybridized to GeneChip with 28,700 rat genes. Using the criterion of fold change > 4 in the expression level, we identified 83 genes that were up-regulated and 89 genes that were down-regulated in oligodendrocytes, and 92 genes that were up-regulated and 86 that were down-regulated in type II astrocytes compared with OPCs. -

1 and ET-3 Inhibit Estrogen and Camp Production by Rat Granulosa Cells in Vitro
209 Endothelin (ET)-1 and ET-3 inhibit estrogen and cAMP production by rat granulosa cells in vitro A E Calogero, N Burrello and A M Ossino Division of Andrology, Department of Internal Medicine, University of Catania, 95123 Catania, Italy (Requests for offprints should be addressed to A E Calogero at Istituto di Medicina Interna e Specialita` Internistiche, Ospedale Garibaldi, Piazza S.M. di Gesu`, 95123 Catania, Italy) Abstract Endothelin (ET)-1 and ET-3, two peptides with a potent and a maximally stimulatory (3 mIU/ml) concentration of vasoconstrictive property, produce a variety of biological FSH. ET-1 and ET-3 dose-dependently suppressed basal effects in different tissues by acting through two different and FSH (1 mIU/ml)-stimulated cAMP production. ET-3 receptors, the ET-1 selective ETA receptor and the non- and SFX-S6c were significantly more potent than ET-1 in selective ETB receptor. An increasing body of literature suppressing estrogen production, suggesting that this effect suggests that ET-1 acts as a paracrine/autocrine regulator was not mediated by the ETA receptor. Indeed, BQ-123, of ovarian function. Indeed, ETB receptors have been a selective ETA receptor antagonist, did not influence identified in rat granulosa cells and ET-1 is a potent the inhibitory effects of ET-1 and ET-3 on basal and inhibitor of progesterone production. In contrast, incon- FSH-stimulated estrogen release. To determine a possible sistent data have been reported about the role of ET-1 on involvement of prostanoids, we evaluated the effects of estrogen production and the effects of ET-3 are not maximally effective concentrations of ET-1 and ET-3 on known. -

Cooperation of Endothelin-1 Signaling with Melanosomes Plays a Role In
© 2015. Published by The Company of Biologists Ltd | Biology Open (2015) 4, 1213-1221 doi:10.1242/bio.011973 RESEARCH ARTICLE Cooperation of endothelin-1 signaling with melanosomes plays a role in developing and/or maintaining human skin hyperpigmentation Daiki Murase1,2,*, Akira Hachiya1,*,‡, Mamiko Kikuchi-Onoe1, Rachel Fullenkamp2, Atsushi Ohuchi1, Takashi Kitahara1, Shigeru Moriwaki1, Tadashi Hase1 and Yoshinori Takema3 ABSTRACT both long-term sun-exposure and chronological aging. Such Skin hyperpigmentation is characterized by increased melanin hyperpigmentation is also thought to be related to the existence of synthesis and deposition that can cause significant psychosocial and uneven skin tones often observed on sun-exposed areas. Regardless of psychological distress. Although several cytokine-receptor signaling the significant psychosocial distress associated with age spots, little is cascades contribute to the formation of ultraviolet B-induced cutaneous known about the detailed mechanisms responsible for them, except for hyperpigmentation, their possible involvement in other types of skin ultraviolet B (UVB)-induced pigmentation, despite the fact that many hyperpigmentation has never been clearly addressed. Since our researchers have tried to identify the melanogenic stimulatory factor(s) continuous studies using skin specimens from more than 30 subjects involved. with ethnic skin diversity emphasized a consistent augmentation in the In the course of UVB-induced pigmentation, three major steps in expression of endothelin-1 (ET-1) and its receptor (Endothelin B the epidermis, melanocyte proliferation, activation of melanin receptor, ET-B) in hyperpigmented lesions, including senile lentigos synthesis and melanosome transfer to keratinocytes, have been (SLs), the precise function of ET-1 signaling was investigated in the reported to be responsible for the increased melanogenesis (Okazaki .