Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Ductoscopy in Patients with Pathological Nipple Discharge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Burns, Surgical Treatment
Philippine College of Surgeons Dear PCS Fellows, We at the PCS Committee on HMO, RVS, & PHIC & The PCS Board of Regents are pleased to announce the Adoption of PAHMOC of our new & revised RVS. We are currently under negotiations with them with regard to the multiplier to be used to arrive at our final professional Fees. Rest assured that we will have a graduated & staggered increase of PF thru the years from what we are currently receiving due to the proposed yearly increments in the multiplier. To those Fellows who haven’t signed the USA (Universal Service Agreement found here in our PCS website) please be reminded to sign and submit to the PCS Secretariat, as only those who did and are in good standing (updated annual dues) will be eligible to avail of the benefits of the new RVS scale. Indeed, we are hoping & looking forward to a merrier 2020 Christmas for our Fellows. Yours truly, FERNANDO L. LOPEZ, MD, FPCS Chairman Noted by: JOSELITO M. MENDOZA, MD, FPCS Regent-in-Charge JOSE ANTONIO M. SALUD, MD, FPCS President For many years now the PCS Committee on HMO & RUV has been compiling, with the assistance of the different surgical subspecialties, a new updated list of RUV for each procedure to replace the existing manual of 2009. This new version not only has a more complete listing of cases but also includes the newly developed procedures particularly for all types of minimally invasive operations. Sometime last year, the Department of Health released Circular 2019-0558 on the Public Access to the Price Information by all Health Providers as required by Section 28.16 of the IRR of the Universal Health Care Act. -

Breast Cancer Screening and Chemoprevention
Management of Breast Diseases Ismail Jatoi Manfred Kaufmann (Eds.) Management of Breast Diseases Dr. Ismail Jatoi Prof. Dr. Manfred Kaufmann Head, Breast Care Center Breast Unit National Naval Medical Center Director, Women’s Hospital Uniformed Services University University of Frankfurt of the Health Sciences Theodor-Stern-Kai 7 4301 Jones Bridge Rd. 60590 Frankfurt Bethesda, MD 20814 Germany USA [email protected] [email protected] ISBN: 978-3-540-69742-8 e-ISBN: 978-3-540-69743-5 DOI: 10.1007/978-3-540-69743-5 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2009934509 © Springer-Verlag Berlin Heidelberg 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and appli- cation contained in this book. -

Mammary Ductoscopy in the Current Management of Breast Disease
Surg Endosc (2011) 25:1712–1722 DOI 10.1007/s00464-010-1465-4 REVIEWS Mammary ductoscopy in the current management of breast disease Sarah S. K. Tang • Dominique J. Twelves • Clare M. Isacke • Gerald P. H. Gui Received: 4 May 2010 / Accepted: 5 November 2010 / Published online: 18 December 2010 Ó Springer Science+Business Media, LLC 2010 Abstract terms ‘‘ductoscopy’’, ‘‘duct endoscopy’’, ‘‘mammary’’, Background The majority of benign and malignant ‘‘breast,’’ and ‘‘intraductal’’ were used. lesions of the breast are thought to arise from the epithe- Results/conclusions Duct endoscopes have become lium of the terminal duct-lobular unit (TDLU). Although smaller in diameter with working channels and improved modern mammography, ultrasound, and MRI have optical definition. Currently, the role of MD is best defined improved diagnosis, a final pathological diagnosis cur- in the management of SND facilitating targeted surgical rently relies on percutaneous methods of sampling breast excision, potentially avoiding unnecessary surgery, and lesions. The advantage of mammary ductoscopy (MD) is limiting the extent of surgical resection for benign disease. that it is possible to gain direct access to the ductal system The role of MD in breast-cancer screening and breast via the nipple. Direct visualization of the duct epithelium conservation surgery has yet to be fully defined. Few allows the operator to precisely locate intraductal lesions, prospective randomized trials exist in the literature, and enabling accurate tissue sampling and providing guidance these would be crucial to validate current opinion, not only to the surgeon during excision. The intraductal approach in the benign setting but also in breast oncologic surgery. -

Prevalence of Breast Cancer in Patients Undergoing
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE Prevalence of breast cancer in patients undergoing microdochectomy for a pathological nipple discharge Dr Chiapo Lesetedi AFRCSI(Dublin), FCS(SA) Student Number: 584356 Department of Surgery University of the Witwatersrand E-mail: [email protected] Cell No: 073 394 2043 Supervisors: Dr Sarah Rayne, MRCS, MMed(Wits), FCS(SA), Department of Surgery, University of the Witwatersrand Dr Deirdré Kruger, BSc, PGCHE, PhD(UK), Department of Surgery, University of the Witwatersrand 18th July 2016 Page 1 of 33 TABLE OF CONTENTS DECLARATION……………………………………………………….………………3 ACKNOWLEDGEMENTS……………………………………………………………4 ABSTRACT…………………………………………………………………………….5 CHAPTER 1: INTRODUCTION…………………………………………...…………7 CHAPTER 2: METHODS……………………………………………………..…….10 CHAPTER 3: RESULTS…………………………………………………………….13 CHAPTER 4: DISCUSSION………………………………………………….…….15 CHAPTER 5: REFERENCES………………………………………………………19 APPENDIX 1: APPROVED PROTOCOL………..………………………………..23 APPENDIX 2: DATA RECORDING SHEET…………………..………………….31 APPENDIX 3: ETHICS CLEARANCE……………………………………….……33 Page 2 of 33 DECLARATION I, Chiapo Lesetedi, declare that this research project is my own work. It is being submitted for the degree of Master of Medicine in Surgery at the University of the Witwatersrand, Johannesburg, South Africa. It is submitted by submissible paper format. It has not been submitted before for any degree or examination at this or any other University. ________________ Dr Chiapo Lesetedi 18th July 2016 Page 3 of 33 ACKNOWLEDGEMENTS I would like to thank my supervisors, Dr Sarah Rayne and Dr Deirdré Kruger, who guided me from the start and continuously encouraged and supported me during the writing up of this research project. They have tirelessly assisted in proof reading the project and Dr Kruger also assisted with data analysis and statistics involved. -

Sub-Areolar Duct Excision (SADE) / Affix Patient Label Microdochectomy
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient Sub-Areolar Duct Excision (SADE) / affix patient label Microdochectomy What is a SADE? SADE is a surgical procedure in which a small portion of the milk ducts from behind the nipple is removed for careful lab analysis to determine the cause of abnormal nipple discharge. Microdochectomy is a procedure to remove a SINGLE discharging duct for the same reason. SADE is the preferred procedure for women who have completed their family and are not anticipating breast feeding in the future. SADE involves the division of all the milk ducts so that breast feeding from that side afterwards is not possible. Microdochectomy is preferred in younger women who wish to be able to breast feed in future. The surgical scar from these two procedures is similar. Why do I need it? In most cases the reasons underlying abnormal nipple discharge are benign (ie non-cancerous), particularly when the breast imaging is normal. However, in a small number of cases with persistent abnormal nipple discharge, the only way to exclude possible underlying early cancerous change is to remove a small portion of the milk ducts for definitive microscopic analysis. Are there any alternatives? py If your clinical examination and breast imaging is normal, then it is commoon practice to wait and watch for a few weeks to see if the discharge settles down on its own. During this pecriod, you are asked to keep diary of discharge. If at the end of this close observation period, thte disc harge continues and there remains enough concern then the only way to exclude serious undnerlying cause for discharge is this surgical procedure. -
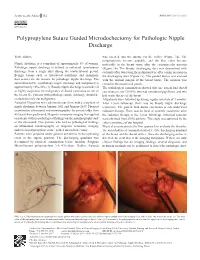
Polypropylene Suture Guided Microdochectomy for Pathologic Nipple Discharge
Letter to the Editor 352 Balkan Med J 2018;35:352-3 Polypropylene Suture Guided Microdochectomy for Pathologic Nipple Discharge To the Editor, was inserted into the ductus via the orifice (Figure 1a). The polypropylene became palpable, and the blue color became Nipple discharge is a complaint of approximately 5% of women. noticeable in the breast tissue after the circumareolar incision Pathologic nipple discharge is defined as unilateral, spontaneous (Figure 1b). The bloody discharging duct was determined with discharge from a single duct during the nonlactational period. certainty after observing the polypropylene after a mini incision on Benign lesions such as intraductal papilloma and mammary the discharging duct (Figure 1c). The guided ductus was excised duct ectasia are the reasons for pathologic nipple discharge. The with the normal margin of the breast tissue. The incision was association between pathologic nipple discharge and malignancy is closed in the anatomical planes. approximately 10%-20% (1). Bloody nipple discharge is considered The pathological examination showed that one patient had ductal as highly suspicious for malignancy or ductal carcinoma in situ of carcinoma in situ (20.0%), two had intraductal papilloma, and two the breast (2). Patients with pathologic nipple discharge should be had cystic disease of the breast. evaluated to rule out malignancy. All patients were followed up during regular intervals of 3 months. A total of 78 patients were admitted to our clinic with a complaint of After 1-year follow-up, there was no bloody nipple discharge nipple discharge between January 2011 and January 2017. Physical recurrence. The patient with ductal carcinoma in situ underwent examination, ultrasound, and mammography (for patients older than radiation therapy. -

Mammary Ductoscopy, Aspiration and Lavage
Medical Coverage Policy Effective Date ............................................. 1/15/2021 Next Review Date ....................................... 1/15/2022 Coverage Policy Number .................................. 0057 Mammary Ductoscopy, Aspiration and Lavage Table of Contents Related Coverage Resources Overview .............................................................. 1 Coverage Policy ................................................... 1 General Background ............................................ 2 Medicare Coverage Determinations .................. 10 Coding/Billing Information .................................. 10 References ........................................................ 10 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may -

Journal of Surgery Leong A, Et Al
Journal of Surgery Leong A, et al. J Surg: JSUR-1154. Research Article DOI: 10.29011/2575-9760. 001154 Variations in Abnormal Nipple Discharge Management in Women- a Systematic Review and Meta-analysis Alison Leong, Alison Johnston, Michael Sugrue* Department of Breast Surgery, Breast Centre North West, Donegal Clinical Research Academy, Letterkenny University Hospital, Done- gal, Ireland *Corresponding author: Michael Sugrue, Department of Breast Surgery, Breast Centre North West, Donegal Clinical Research Academy, Letterkenny University Hospital, Donegal, Ireland. Tel: +353749188823; Fax: +353749188816; Email: michael.sugrue@ hse.ie Citation: Leong A, Johnston A, Sugrue M (2018) Variations in Abnormal Nipple Discharge Management in Women- a Systematic Review and Meta-analysis. J Surg: JSUR-1154. DOI: 10.29011/2575-9760. 001154 Received Date: 13 July, 2018; Accepted Date: 19 July, 2018; Published Date: 26 July, 2018 Abstract Nipple discharge accounts for 5% of referrals to breast units; breast cancer in image negative nipple discharge patients varies from 0 to 21%. This systematic review and meta-analysis determined variability in breast cancer rates in nipple discharge patients, diagnostic accuracy of modalities and surgery rates. An ethically approved meta-analysis was conducted using data- bases PubMed, EMBASE, and Cochrane Library from January 2000 to July 2015. For the breast cancer rates’ review, studies were excluded if no clinical follow-up data was available. For the diagnostic accuracy meta-analysis, studies were excluded if there was no reference standard, or the number of true and false positives and negatives were not known. Pooled sensitivities were determined using Mantel-Haenszel method. For the surgery rates’ review, only studies with consecutive nipple discharge patients were included. -

TABLE of SURGICAL PROCEDURES (Updated As of 2 Jan 2019)
Ministry of Health TABLE OF SURGICAL PROCEDURES (Updated as of 2 Jan 2019) 1 TABLE OF CONTENTS SA - Integumentary ........................................................................... 3 SB - Musculoskeletal ....................................................................... 10 SC - Respiratory .............................................................................. 27 SD - Cardiovascular ........................................................................ 30 SE - Hemic & Lymphatic ................................................................. 37 SF - Digestive .................................................................................. 39 SG - Urinary .................................................................................... 51 SH - Male Genital ............................................................................ 55 SI - Female Genital ......................................................................... 57 SJ - Endocrine ................................................................................. 64 SK - Nervous ................................................................................... 65 SL - Eye ........................................................................................... 72 SM - ENT ......................................................................................... 78 The Table of Surgical Procedures (TOSP) is an exhaustive list of procedures with table ranking 1A to 7C, for which MediSave / MediShield Life can be claimed. Any procedures not listed -
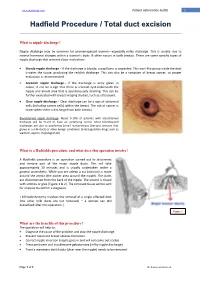
Hadfield Procedure / Total Duct Excision
www.drsoobrah.com Patient information leaflet 1 Hadfield Procedure / Total duct excision What is nipple discharge? Nipple discharge may be common for premenopausal women—especially milky discharge. This is usually due to normal hormonal changes within a woman’s body. It often occurs in both breasts. There are some specific types of nipple discharge that warrant closer evaluation: Bloody nipple discharge – If the discharge is bloody, a papilloma is suspected. This wart-like group inside the duct irritates the tissue, producing the reddish discharge. This can also be a symptom of breast cancer, so proper evaluation is recommended. Greenish nipple discharge – If the discharge is army green in colour, it can be a sign that there is a breast cyst underneath the nipple and areola area that is spontaneously draining. This can be further evaluated with breast imaging studies, such as ultrasound. Clear nipple discharge – Clear discharge can be a sign of abnormal cells (including cancer cells) within the breast. The risk of cancer is lower when there is discharge from both breasts. Bloodstained nipple discharge: About 5-10% of patients with bloodstained discharge will be found to have an underlying cancer. Most bloodstained discharges are due to papillomas [small noncancerous (benign) tumours that grows in a milk duct] or other benign conditions (anticoagulation drugs such as warfarin, aspirin, clopidogrel etc). What is a Hadfields procedure and what does this operation involve? A Hadfields procedure is an operation carried out to disconnect and remove part of the major nipple ducts. This will take approximately 30 minutes and is usually undertaken under a general anaesthetic. -

Endoscopic Appearance and Clinicopathological Character of Breast Cancer
ANTICANCER RESEARCH 31: 3517-3520 (2011) Endoscopic Appearance and Clinicopathological Character of Breast Cancer DAIGO YAMAMOTO1, YU TSUBOTA1, HIDEYUKI YOSHIDA2, SAYAKA KANEMATSU2, NORIKO SUEOKA1, YOSHIKO UEMURA3, KANJI TANAKA4 and A-HON KWON1 1Department of Surgery, Kansai Medical University, Hirakata, Osaka, Japan; 2Department of Surgery, Kansai Medical University, Kohri, Osaka, Japan; 3Clinical Pathology, Kansai Medical University, Hirakata, Osaka, Japan; 4Breast Unit, Ribbon-rose Clinic, Osaka, Japan Abstract. Background: The Japanese Association of Nipple discharge is a common complaint among women. In Mammary Ductoscopy proposed a classification system based studies to date (1-4), the etiology is usually benign: on the objective endoscopic appearance of intraductal lesions. papilloma is the most common cause (40-70%), followed by This system includes four categories: solitary polypoid, adenomatous or papillary epithelial proliferation (14%). multiple polypoid, superficial, and combined type. However, However, 1-23% of women with pathologic nipple discharge previous studies did not adequately compare endoscopic are diagnosed with breast cancer or ductal carcinoma in situ; findings with histological findings and the prognosis. Patients therefore, further investigations including surgical duct and Methods: One hundred and ten patients with nipple excision are generally recommended. discharge who had intraductal tumors were identified by Fiberoptic ductoscopy is an emerging technique mammary ductoscopy, and 25 breast cancer patients were facilitating direct visual access to the ductal system of the identified from our database of records between 2001 and breast through nipple orifice cannulation and exploration (5- 2008. The clinicopathological data and outcomes of these 7). Ductoscopy allows the direct observation of lesions and is patients were then reviewed. Results: Lesions in 25 breast not simply indirect shadowing. -

Nipple Discharge K
Current Management of Nipple Discharge 6 Richard J. Gray and Barbara A. Pockaj Background and Pathophysiology by hyperprolactinemia, which may be secondary to medications, endocrine tumors (i.e., pituitary Nipple discharge is a relatively common complaint, adenoma), endocrine abnormalities, or a variety with a reported incidence of 2–5 % [ 1 ] and occur- of other medical conditions [16 ]. ring among 10–50 % of patients with benign breast Pathologic nipple discharge is characterized disease [ 1 , 2 ]. Typically, the primary concern and by a unilateral, spontaneous, persistent dis- initial fear of patients who experience nipple dis- charge from a single duct. Pathologic discharge charge is whether it is due to an underlying breast is not necessarily caused by an underlying car- cancer. The risk of carcinoma among those with cinoma, and in fact, most pathologic nipple nipple discharge has been reported to be between discharge is a result of a periductal mastitis, 6 and 21 % [ 2 – 10 ], with some reports including duct ectasia, or benign intraductal papilloma. only those patients undergoing an operation, while Periductal mastitis typically produces multi- others do not [ 3 , 7 – 10 ]. Nipple discharge can be colored, sticky discharge. Duct ectasia is the separated into categories of normal milk produc- result of increased glandular secretions by the tion (lactation), galactorrhea (physiologic nipple lactiferous ducts and results in multi-duct, col- discharge), or pathologic nipple discharge based ored discharge that can often be bilateral. on the characteristics of presentation [ 11 ]. Intraductal papilloma generally produces Lactation occurs as early as the second trimes- serous or bloody discharge from a single duct. ter of pregnancy and can continue for up to Other related nipple abnormalities that the cli- 2 years after delivery or cessation of breastfeed- nician should be aware of that can produce ing [ 12 ].