Journal of Surgery Leong A, Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Burns, Surgical Treatment
Philippine College of Surgeons Dear PCS Fellows, We at the PCS Committee on HMO, RVS, & PHIC & The PCS Board of Regents are pleased to announce the Adoption of PAHMOC of our new & revised RVS. We are currently under negotiations with them with regard to the multiplier to be used to arrive at our final professional Fees. Rest assured that we will have a graduated & staggered increase of PF thru the years from what we are currently receiving due to the proposed yearly increments in the multiplier. To those Fellows who haven’t signed the USA (Universal Service Agreement found here in our PCS website) please be reminded to sign and submit to the PCS Secretariat, as only those who did and are in good standing (updated annual dues) will be eligible to avail of the benefits of the new RVS scale. Indeed, we are hoping & looking forward to a merrier 2020 Christmas for our Fellows. Yours truly, FERNANDO L. LOPEZ, MD, FPCS Chairman Noted by: JOSELITO M. MENDOZA, MD, FPCS Regent-in-Charge JOSE ANTONIO M. SALUD, MD, FPCS President For many years now the PCS Committee on HMO & RUV has been compiling, with the assistance of the different surgical subspecialties, a new updated list of RUV for each procedure to replace the existing manual of 2009. This new version not only has a more complete listing of cases but also includes the newly developed procedures particularly for all types of minimally invasive operations. Sometime last year, the Department of Health released Circular 2019-0558 on the Public Access to the Price Information by all Health Providers as required by Section 28.16 of the IRR of the Universal Health Care Act. -

Evicore Breast Imaging Guidelines
CLINICAL GUIDELINES Breast Imaging Guidelines Version 2.0 Effective September 1, 2021 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2021 eviCore healthcare. All rights reserved. Breast Imaging Guidelines V2.0 Breast Imaging Guidelines Abbreviations for Breast Guidelines 3 BR-Preface1: General Considerations 5 BR-1: Breast Ultrasound 8 BR-2: MRI Breast 10 BR-3: Breast Reconstruction 12 BR-4: MRI Breast is NOT Indicated 13 BR-5: MRI Breast Indications 14 BR-6: Nipple Discharge/Galactorrhea 18 BR-7: Breast Pain (Mastodynia) 19 BR-8: Alternative Breast Imaging Approaches 20 BR-9: Suspected Breast Cancer in Males 22 BR-10: Breast Evaluation in Pregnant or Lactating Females 23 ______________________________________________________________________________________________________ -

Biopsy Needles and Drainage Catheters
Needles & Accessories | Catheters & Accessories Dilation & Accessories Spinal & Accessories | Implantable & Accessories Product Catalog ISO 13485 & ISO 9001 Certified Company Rev. 01 - 2019/03 About ADRIA Srl. Adria is a worldwide leader in developing, manufacturing and marketing healthcare products. The main focus is on Radiology, Oncology, Urology, Gynecology and Surgery . Adria' s corporate headquarter is based in Italy, it is ISO Certified and products are CE . marked. Adria was incorporated more than 20 years ago in Bologna , where the corporate headquarter and production plant is located. Adria is leader in developing and manufacturing healthcare products and keeps the status to be one of the first companies aimed to develop single patient use biopsy needles and drainage catheters. Over the time, thanks to the experience of specialized doctors and engineers , Adria product range and quality have been progressively enhanced, involving and developing the spine treatment line. Nowadays Adria has a worldwide presence . Reference markets are France, Spain, Turkey and products are distributed in more than 50 countries, through a large and qualified network of dealers. Since far off the very beginning, many things have changed, but Adria' s philosophy and purpose have always remained unchanged: helping healthcare providers to fulfill their mission of caring for patients. Table of Contents Needles & Accessories …………………………………………….....…...3 Catheters & Accessories ……………………….………………..…...…...18 Dilation & Accessories ……………………………………………...…...25 Spinal & Accessories ……………………………………………...…...30 Implantables & Accessories……………………………………………...…...35 Needles & Accessories HYSTO SYSTEM Automatic Biopsy Instrument……………………….……….…….. 4 HYSTO SYSTEM II Automatic Biopsy Instrument…………………….……….……...5 SAMPLE MASTER Semi-Automatic Biopsy Instrument…………………………….. 6 HYSTO-ONE Automatic Reusable Biopsy Instrument & MDA Biopsy Needle ....... 7 HYSTO-TWO Automatic Reusable Biopsy Instrument & MDS Biopsy Needle…... -

Evaluation of Nipple Discharge
New 2016 American College of Radiology ACR Appropriateness Criteria® Evaluation of Nipple Discharge Variant 1: Physiologic nipple discharge. Female of any age. Initial imaging examination. Radiologic Procedure Rating Comments RRL* Mammography diagnostic 1 See references [2,4-7]. ☢☢ Digital breast tomosynthesis diagnostic 1 See references [2,4-7]. ☢☢ US breast 1 See references [2,4-7]. O MRI breast without and with IV contrast 1 See references [2,4-7]. O MRI breast without IV contrast 1 See references [2,4-7]. O FDG-PEM 1 See references [2,4-7]. ☢☢☢☢ Sestamibi MBI 1 See references [2,4-7]. ☢☢☢ Ductography 1 See references [2,4-7]. ☢☢ Image-guided core biopsy breast 1 See references [2,4-7]. Varies Image-guided fine needle aspiration breast 1 Varies *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Pathologic nipple discharge. Male or female 40 years of age or older. Initial imaging examination. Radiologic Procedure Rating Comments RRL* See references [3,6,8,10,13,14,16,25- Mammography diagnostic 9 29,32,34,42-44,71-73]. ☢☢ See references [3,6,8,10,13,14,16,25- Digital breast tomosynthesis diagnostic 9 29,32,34,42-44,71-73]. ☢☢ US is usually complementary to mammography. It can be an alternative to mammography if the patient had a recent US breast 9 mammogram or is pregnant. See O references [3,5,10,12,13,16,25,30,31,45- 49]. MRI breast without and with IV contrast 1 See references [3,8,23,24,35,46,51-55]. -

Breast Cancer Screening and Chemoprevention
Management of Breast Diseases Ismail Jatoi Manfred Kaufmann (Eds.) Management of Breast Diseases Dr. Ismail Jatoi Prof. Dr. Manfred Kaufmann Head, Breast Care Center Breast Unit National Naval Medical Center Director, Women’s Hospital Uniformed Services University University of Frankfurt of the Health Sciences Theodor-Stern-Kai 7 4301 Jones Bridge Rd. 60590 Frankfurt Bethesda, MD 20814 Germany USA [email protected] [email protected] ISBN: 978-3-540-69742-8 e-ISBN: 978-3-540-69743-5 DOI: 10.1007/978-3-540-69743-5 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2009934509 © Springer-Verlag Berlin Heidelberg 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and appli- cation contained in this book. -

Ultrasound, Elastography and MRI Mammography
EAS Journal of Radiology and Imaging Technology Abbreviated Key Title: EAS J Radiol Imaging Technol ISSN 2663-1008 (Print) & ISSN: 2663-7340 (Online) Published By East African Scholars Publisher, Kenya Volume-1 | Issue-2 | Mar-Apr-2019 | Research Article Ultrasound, Elastography and MRI Mammography Correlation in Breast Pathologies (A Study of 50 Cases) Dr Hiral Parekh.1, Dr Lata Kumari.2, Dr Dharmesh Vasavada.3 1Professor, Department of Radiodiagnosis M P Shah Government Medical College Jamnagar, Gujarat, India 2Resident Doctor in Radiodiagnosis Department of Radiodiagnosis M P Shah Government Medical College Jamnagar, Gujarat, India 3Professor, Department of Surgery M P Shah Government Medical College Jamnagar, Gujarat, India *Corresponding Author Dr Dharmesh Vasavada Abstract: Introduction: The purpose of this study is to investigate the value of MRI in comparison to US and mammography in diagnosis of breast lesions. MRI is ideal for breast imaging due to its ability to depict excellent soft tissue contrast. Methods: This study of 50 cases was conducted in the department of Radiodiagnosis, Guru Gobinsingh Government Hospital, M P Shah Government Medical College, Jamnagar, Gujarat, India. All 50 cases having or suspected to have breast lesions were chosen at random among the indoor and outdoor patients referred to the Department of Radiodiagnosis for imaging. Discussion: In the present study the results of sonoelastography were compared with MRI. The malignant masses were the commonest and the mean age of patients with malignant masses in our study was 45 years, which is in consistent with Park‟s statement that the mean age of breast cancer occurrence is about 42 years in India3. -

Diagnostic Accuracy of Shear Wave Elastography for Prediction of Breast Malignancy in Patients with Pathological Nipple Discharge
Open Access Research BMJ Open: first published as 10.1136/bmjopen-2015-008848 on 22 January 2016. Downloaded from Diagnostic accuracy of shear wave elastography for prediction of breast malignancy in patients with pathological nipple discharge Xiaobo Guo,1 Ying Liu,1 Wanhu Li2 To cite: Guo X, Liu Y, Li W. ABSTRACT Strengths and limitations of this study Diagnostic accuracy of shear Objectives: Pathological nipple discharge (PND) may wave elastography for indicate malignant breast lesions. As the role of shear ▪ prediction of breast Diagnostic accuracy of shear wave elastography wave elastography (SWE) in predicting these malignant malignancy in patients with (SWE) for detecting malignancy of patients with pathological nipple discharge. lesions has not yet been evaluated, we aim to evaluate PND has rarely been studied. BMJ Open 2016;6:e008848. the diagnostic value of SWE for this condition. ▪ For the first time, this study tested diagnostic doi:10.1136/bmjopen-2015- Design: Prospective diagnostic accuracy study accuracy of a synthesised measurement of quali- 008848 comparing a combination of qualitative and quantitative tative and quantitative measures of SWE for measurements of SWE (index test) to a ductoscopy detecting malignancy in patients with PND. ▸ Prepublication history for and microdochectomy for histological diagnosis ▪ Limitations include the fact that the weight of this paper is available online. (reference test). each measurement in the synthesised score was To view these files please Setting: Fuzhou General Hospital of Nanjing military assigned evenly and the surgeon was not visit the journal online command. blinded. (http://dx.doi.org/10.1136/ Participants: A total of 379 patients with PND were bmjopen-2015-008848). -

Mammary Ductoscopy in the Current Management of Breast Disease
Surg Endosc (2011) 25:1712–1722 DOI 10.1007/s00464-010-1465-4 REVIEWS Mammary ductoscopy in the current management of breast disease Sarah S. K. Tang • Dominique J. Twelves • Clare M. Isacke • Gerald P. H. Gui Received: 4 May 2010 / Accepted: 5 November 2010 / Published online: 18 December 2010 Ó Springer Science+Business Media, LLC 2010 Abstract terms ‘‘ductoscopy’’, ‘‘duct endoscopy’’, ‘‘mammary’’, Background The majority of benign and malignant ‘‘breast,’’ and ‘‘intraductal’’ were used. lesions of the breast are thought to arise from the epithe- Results/conclusions Duct endoscopes have become lium of the terminal duct-lobular unit (TDLU). Although smaller in diameter with working channels and improved modern mammography, ultrasound, and MRI have optical definition. Currently, the role of MD is best defined improved diagnosis, a final pathological diagnosis cur- in the management of SND facilitating targeted surgical rently relies on percutaneous methods of sampling breast excision, potentially avoiding unnecessary surgery, and lesions. The advantage of mammary ductoscopy (MD) is limiting the extent of surgical resection for benign disease. that it is possible to gain direct access to the ductal system The role of MD in breast-cancer screening and breast via the nipple. Direct visualization of the duct epithelium conservation surgery has yet to be fully defined. Few allows the operator to precisely locate intraductal lesions, prospective randomized trials exist in the literature, and enabling accurate tissue sampling and providing guidance these would be crucial to validate current opinion, not only to the surgeon during excision. The intraductal approach in the benign setting but also in breast oncologic surgery. -

Prevalence of Breast Cancer in Patients Undergoing
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE Prevalence of breast cancer in patients undergoing microdochectomy for a pathological nipple discharge Dr Chiapo Lesetedi AFRCSI(Dublin), FCS(SA) Student Number: 584356 Department of Surgery University of the Witwatersrand E-mail: [email protected] Cell No: 073 394 2043 Supervisors: Dr Sarah Rayne, MRCS, MMed(Wits), FCS(SA), Department of Surgery, University of the Witwatersrand Dr Deirdré Kruger, BSc, PGCHE, PhD(UK), Department of Surgery, University of the Witwatersrand 18th July 2016 Page 1 of 33 TABLE OF CONTENTS DECLARATION……………………………………………………….………………3 ACKNOWLEDGEMENTS……………………………………………………………4 ABSTRACT…………………………………………………………………………….5 CHAPTER 1: INTRODUCTION…………………………………………...…………7 CHAPTER 2: METHODS……………………………………………………..…….10 CHAPTER 3: RESULTS…………………………………………………………….13 CHAPTER 4: DISCUSSION………………………………………………….…….15 CHAPTER 5: REFERENCES………………………………………………………19 APPENDIX 1: APPROVED PROTOCOL………..………………………………..23 APPENDIX 2: DATA RECORDING SHEET…………………..………………….31 APPENDIX 3: ETHICS CLEARANCE……………………………………….……33 Page 2 of 33 DECLARATION I, Chiapo Lesetedi, declare that this research project is my own work. It is being submitted for the degree of Master of Medicine in Surgery at the University of the Witwatersrand, Johannesburg, South Africa. It is submitted by submissible paper format. It has not been submitted before for any degree or examination at this or any other University. ________________ Dr Chiapo Lesetedi 18th July 2016 Page 3 of 33 ACKNOWLEDGEMENTS I would like to thank my supervisors, Dr Sarah Rayne and Dr Deirdré Kruger, who guided me from the start and continuously encouraged and supported me during the writing up of this research project. They have tirelessly assisted in proof reading the project and Dr Kruger also assisted with data analysis and statistics involved. -

Sub-Areolar Duct Excision (SADE) / Affix Patient Label Microdochectomy
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient Sub-Areolar Duct Excision (SADE) / affix patient label Microdochectomy What is a SADE? SADE is a surgical procedure in which a small portion of the milk ducts from behind the nipple is removed for careful lab analysis to determine the cause of abnormal nipple discharge. Microdochectomy is a procedure to remove a SINGLE discharging duct for the same reason. SADE is the preferred procedure for women who have completed their family and are not anticipating breast feeding in the future. SADE involves the division of all the milk ducts so that breast feeding from that side afterwards is not possible. Microdochectomy is preferred in younger women who wish to be able to breast feed in future. The surgical scar from these two procedures is similar. Why do I need it? In most cases the reasons underlying abnormal nipple discharge are benign (ie non-cancerous), particularly when the breast imaging is normal. However, in a small number of cases with persistent abnormal nipple discharge, the only way to exclude possible underlying early cancerous change is to remove a small portion of the milk ducts for definitive microscopic analysis. Are there any alternatives? py If your clinical examination and breast imaging is normal, then it is commoon practice to wait and watch for a few weeks to see if the discharge settles down on its own. During this pecriod, you are asked to keep diary of discharge. If at the end of this close observation period, thte disc harge continues and there remains enough concern then the only way to exclude serious undnerlying cause for discharge is this surgical procedure. -

Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Ductoscopy in Patients with Pathological Nipple Discharge
Systematic review Systematic review and meta-analysis of the diagnostic accuracy of ductoscopy in patients with pathological nipple discharge L. Waaijer1,J.M.Simons1,I.H.M.BorelRinkes1,P.J.vanDiest2,H.M.Verkooijen3 and A. J. Witkamp1 Departments of 1Surgery and 2Pathology and 3Imaging Division, University Medical Centre Utrecht, Utrecht, The Netherlands Correspondence to: Ms L. Waaijer, Department of Surgery, University Medical Centre Utrecht, PO Box 85500, G04.228, 3508 GA Utrecht, The Netherlands (e-mail: [email protected]) Background: Invasive surgery remains the standard for diagnosis of pathological nipple discharge (PND). Only a minority of patients with nipple discharge and an unsuspicious finding on conventional breast imaging have cancer. Ductoscopy is a minimally invasive alternative for evaluation of PND. This systematic review and meta-analysis was designed to evaluate the diagnostic accuracy of ductoscopy in patients with PND. Methods: A systematic search of electronic databases for studies addressing ductoscopy in patients with PND was conducted. Two classification systems were assessed. Forany DS , all visualized ductoscopic abnormalities were classified as positive, whereas forsusp DS , only suspicious findings were considered positive. After checking heterogeneity, pooled sensitivity and specificity of DSany and DSsusp were calculated. Results: The search yielded 4642 original citations, of which 20 studies were included in the review. Malignancy rates varied from 0 to 27 per cent. Twelve studies, including 1994 patients, were eligible for meta-analysis. Pooled sensitivity and specificity of DSany were 94 (95 per cent c.i. 88 to 97) per cent and 47 (44 to 49) per cent respectively. Pooled sensitivity and specificity ofsusp DS were 50 (36 to 64) and 83 (81 to 86) per cent respectively. -
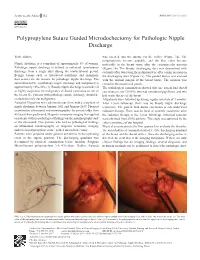
Polypropylene Suture Guided Microdochectomy for Pathologic Nipple Discharge
Letter to the Editor 352 Balkan Med J 2018;35:352-3 Polypropylene Suture Guided Microdochectomy for Pathologic Nipple Discharge To the Editor, was inserted into the ductus via the orifice (Figure 1a). The polypropylene became palpable, and the blue color became Nipple discharge is a complaint of approximately 5% of women. noticeable in the breast tissue after the circumareolar incision Pathologic nipple discharge is defined as unilateral, spontaneous (Figure 1b). The bloody discharging duct was determined with discharge from a single duct during the nonlactational period. certainty after observing the polypropylene after a mini incision on Benign lesions such as intraductal papilloma and mammary the discharging duct (Figure 1c). The guided ductus was excised duct ectasia are the reasons for pathologic nipple discharge. The with the normal margin of the breast tissue. The incision was association between pathologic nipple discharge and malignancy is closed in the anatomical planes. approximately 10%-20% (1). Bloody nipple discharge is considered The pathological examination showed that one patient had ductal as highly suspicious for malignancy or ductal carcinoma in situ of carcinoma in situ (20.0%), two had intraductal papilloma, and two the breast (2). Patients with pathologic nipple discharge should be had cystic disease of the breast. evaluated to rule out malignancy. All patients were followed up during regular intervals of 3 months. A total of 78 patients were admitted to our clinic with a complaint of After 1-year follow-up, there was no bloody nipple discharge nipple discharge between January 2011 and January 2017. Physical recurrence. The patient with ductal carcinoma in situ underwent examination, ultrasound, and mammography (for patients older than radiation therapy.