NMR Studies of Phosphorus Ligand Complexes of Silver and Cobalt Steven Mark Socol Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet According to 1907/2006/EC, Article 31 Printing Date 17.04.2015 Version Number 2 Revision: 17.04.2015
Page 1/10 Safety data sheet according to 1907/2006/EC, Article 31 Printing date 17.04.2015 Version number 2 Revision: 17.04.2015 * SECTION 1: Identification of the substance/mixture and of the company/undertaking · 1.1 Product identifier · Trade name: Arcuplat Schnellversilberung m. 30g Ag/l Arcuplat silver bath with 30 g Ag/l · 1.2 Relevant identified uses of the substance or mixture and uses advised against No further relevant information available. · Application of the substance / the mixture Galvanic bath · 1.3 Details of the supplier of the safety data sheet · Manufacturer/Supplier: Wieland Dental + Technik GmbH & Co. KG Lindenstr. 2 75175 Pforzheim Telefon +49 (0) 7231-37050, Telefax +49 (0) 7231-357959 · Further information obtainable from: [email protected] · 1.4 Emergency telephone number: GIZ-Nord, Göttingen, Germany +49 551 19240 Member of EPECS Network * SECTION 2: Hazards identification · 2.1 Classification of the substance or mixture · Classification according to Regulation (EC) No 1272/2008 GHS06 skull and crossbones Acute Tox. 2 H300 Fatal if swallowed. Acute Tox. 1 H310 Fatal in contact with skin. Acute Tox. 3 H331 Toxic if inhaled. GHS05 corrosion Skin Corr. 1C H314 Causes severe skin burns and eye damage. GHS09 environment Aquatic Chronic 2 H411 Toxic to aquatic life with long lasting effects. · Classification according to Directive 67/548/EEC or Directive 1999/45/EC T+; Very toxic R26/27/28: Very toxic by inhalation, in contact with skin and if swallowed. C; Corrosive R34: Causes burns. N; Dangerous for the environment R51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. -

Approach to Site-Selective Protein Modification JUSTIN M
A “Tag-and-Modify” Approach to Site-Selective Protein Modification JUSTIN M. CHALKER, GONC-ALO J. L. BERNARDES, AND BENJAMIN G. DAVIS* Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford OX1 3TA, United Kingdom RECEIVED ON FEBRUARY 27, 2011 CONSPECTUS ovalent modification can expand a protein's functional capacity. Fluorescent or radioactive labeling, for instance, allows C imaging of a protein in real time. Labeling with an affinity probe enables isolation of target proteins and other interacting molecules. At the other end of this functional spectrum, protein structures can be naturally altered by enzymatic action. ProteinÀprotein interactions, genetic regulation, and a range of cellular processes are under the purview of these post- translational modifications. The ability of protein chemists to install these covalent additions selectively has been critical for elucidating their roles in biology. Frequently the transformations must be applied in a site-specific manner, which demands the most selective chemistry. In this Account, we discuss the development and application of such chemistry in our laboratory. A centerpiece of our strategy is a “tag-and-modify” approach, which entails sequential installation of a uniquely reactive chemical group into the protein (the “tag”) and the selective or specific modification of this group. The chemical tag can be a natural or unnatural amino acid residue. Of the natural residues, cysteine is the most widely used as a tag. Early work in our program focused on selective disulfide formation in the synthesis of glycoproteins. For certain applications, the susceptibility of disulfides to reduction was a limitation and prompted the development of several methods for the synthesis of more stable thioether modifications. -

United States Patent Office Patented Apr
3,803,252 United States Patent Office Patented Apr. 9, 1974 1. 2 3,803,252 in which R is as hereinbefore defined and Y represents PROCESS FOR THE PREPARATION OF CAROTENOD COMPOUNDS a hydrogen atom or a retinyl radical. This formula may Pierre Chabardes, Lyon, and Marc Julia, Paris, France, represent sterically pure products, or mixtures of differ assignors to Rhone-Poulenc S.A., Paris, France ent isomers. 5 The unsubstituted sulphones used as starting mate No Drawing. Filed May 17, 1972, Ser. No. 254,103 rials have the Formula I in which Y represents a hy Claims priority, applicitFrance, May 19, 1971, drogen atom; they are called herein retinyl-Sulphones. Int, C. C07c9 13/00 They can be prepared by known methods for the prep U.S. C. 260-666 C 9 Claims aration of sulphones. A particularly advantageous method O consists of reacting an alkali metal sulphinate of the formula RSOM, in which R represents alkyl, aryl, alkyl ABSTRACT OF THE DISCLOSURE aryl or aralkyl, preferably phenyl or tolyl, and M rep Caroteine compounds e.g. g-caroteine, are prepared by resents an alkali metal, with retinol or a retinol ester reacting a sulphone Ret-SO-R, in which Ret repre of an inorganic or organic acid, such as retinyl chloride sents retinyl or substituted retinyl and R represents a 15 or bromide, or retinyl formate or acetate. They can also hydrocarbon radical, in the presence of an alkaline be prepared by reacting a sulphinic acid RSOH with reagent with an ester Ret-X, in which X represents an retinol, it being possible to form this acid in situ, if re acid residue, and then desulphonating the product, for quired, from a metal sulphinate in an acid medium. -

Ncomms12703.Pdf
ARTICLE Received 17 Feb 2016 | Accepted 26 Jul 2016 | Published 2 Sep 2016 DOI: 10.1038/ncomms12703 OPEN Chemoselective synthesis and analysis of naturally occurring phosphorylated cysteine peptides Jordi Bertran-Vicente1, Martin Penkert1,2, Olaia Nieto-Garcia1,2, Jean-Marc Jeckelmann3, Peter Schmieder1, Eberhard Krause1 & Christian P. R. Hackenberger1,2 In contrast to protein O-phosphorylation, studying the function of the less frequent N- and S-phosphorylation events have lagged behind because they have chemical features that prevent their manipulation through standard synthetic and analytical methods. Here we report on the development of a chemoselective synthetic method to phosphorylate Cys side-chains in unprotected peptides. This approach makes use of a reaction between nucleophilic phosphites and electrophilic disulfides accessible by standard methods. We achieve the stereochemically defined phosphorylation of a Cys residue and verify the modification using electron-transfer higher-energy dissociation (EThcD) mass spectrometry. To demonstrate the use of the approach in resolving biological questions, we identify an endogenous Cys phosphorylation site in IICBGlc, which is known to be involved in the carbohydrate uptake from the bacterial phosphotransferase system (PTS). This new chemical and analytical approach finally allows further investigating the functions and significance of Cys phosphorylation in a wide range of crucial cellular processes. 1 Leibniz-Institut fu¨r Molekulare Pharmakologie (FMP), Robert-Ro¨ssle-Str. 10, D-13125 Berlin, Germany. 2 Humboldt-Universita¨t zu Berlin, Institut fu¨r Chemie, Brook-Taylor-Str. 2, D-12489 Berlin, Germany. 3 Institute of Biochemistry and Molecular Medicine, University of Bern, 3012 Bern, Switzerland. Correspondence and requests for materials should be addressed to C.P.R.H. -

United States Patent (19) 11 4,311,652 Abramson Et Al
United States Patent (19) 11 4,311,652 Abramson et al. 45 Jan. 19, 1982 54 ARBUZOV REACTIONS EMPLOYING AN 56) References Cited ALEPHATIC SOLVENT U.S. PATENT DOCUMENTS 3,483,279 12/1969 Davis et al. ......................... 260/969 75 Inventors: Alan Abramson, White Plains; 3,699,193 10/1972 Melton ..... ... 260/969 Edward D. Weil, 3,776,984 12/1973 Ratts ................................... 260/969 Hastings-on-Hudson, both of N.Y. Primary Examinter-Anton H. Sutto Attorney, Agent, or Firm-Vivienne T. White 73 Assignee: Stauffer Chemical Company, 57 ABSTRACT Westport, Conn. An improved process for phosphite rearrangement wherein the phosphite is rearranged to the phospho nate. The improvement comprises conducting the phos 21 Appl. No.: 154,170 phite rearrangement reaction in an aliphatic solvent which is miscible with the reactants at reaction temper atures, and immiscible with the product at lower tem 22 Filed: May 28, 1980 peratures. Increased yields of the phosphonate product are obtained without the need for additional distillation 51 int. Cl. ................................................ C07F 9/40 for solvent separation. 52 U.S. C. ................ ... 260/969; 260/990 58 Field of Search ................................ 260/969, 990 12 Claims, No Drawings 4,311,652 3 4. The advantage of conducting the rearrangement in a process for rearranging tris(2-chloroethyl) phosphite. solvent were found to be a more easily controlled exo The invention is directed to phosphite rearrangements, therm due to the heating and reflux of the solvent which wherein the novel process comprises conducting the removes the heat of reaction, and inhibiting the degra rearrangement process in an essentially aliphatic Solvent dation of the phosphonate product produced. -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Organophosphate Insecticides
CHAPTER 4 HIGHLIGHTS Organophosphate Insecticides Acts through phosphorylation of the acetylcholinesterase enzyme Since the removal of organochlorine insecticides from use, organophosphate at nerve endings insecticides have become the most widely used insecticides available today. More Absorbed by inhalation, than forty of them are currently registered for use and all run the risk of acute ingestion, and skin and subacute toxicity. Organophosphates are used in agriculture, in the home, penetration in gardens, and in veterinary practice. All apparently share a common mecha- Muscarinic, nicotinic & CNS nism of cholinesterase inhibition and can cause similar symptoms. Because they effects share this mechanism, exposure to the same organophosphate by multiple routes or to multiple organophosphates by multiple routes can lead to serious additive Signs and Symptoms: toxicity. It is important to understand, however, that there is a wide range of Headache, hypersecretion, toxicity in these agents and wide variation in cutaneous absorption, making muscle twitching, nausea, specific identification and management quite important. diarrhea Respiratory depression, seizures, loss of consciousness Toxicology Miosis is often a helpful Organophosphates poison insects and mammals primarily by phosphory- diagnostic sign lation of the acetylcholinesterase enzyme (AChE) at nerve endings. The result is a loss of available AChE so that the effector organ becomes overstimulated by Treatment: the excess acetylcholine (ACh, the impulse-transmitting substance) in the nerve Clear airway, improve tissue ending. The enzyme is critical to normal control of nerve impulse transmission oxygenation from nerve fibers to smooth and skeletal muscle cells, glandular cells, and Administer atropine sulfate autonomic ganglia, as well as within the central nervous system (CNS). -

Synthetic, Sterochemical, and Electronic Studies of Organophosphorous Ligands and Their Transition Metal Complexes James Timothy Spencer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1984 Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes James Timothy Spencer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Spencer, James Timothy, "Synthetic, sterochemical, and electronic studies of organophosphorous ligands and their transition metal complexes " (1984). Retrospective Theses and Dissertations. 7727. https://lib.dr.iastate.edu/rtd/7727 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

Asymmetric Synthesis of Organophosphates and Their
ASYMMETRIC SYNTHESIS OF ORGANOPHOSPHATES AND THEIR DERIVATIVES Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Chemistry By Batool J. Murtadha Dayton, Ohio May 2020 ASYMMETRIC SYNTHESIS OF ORGANOPHOSPHATES AND THEIR DERIVATIVES Name: Murtadha, Batool J. APPROVED BY: __________________________________ Jeremy Erb, Ph.D. Research Advisor Assistant Professor Department of Chemistry University of Dayton ___________________________________ Vladimir Benin, Ph.D. Professor of Chemistry Department of Chemistry University of Dayton ___________________________________ Justin C. Biffinger, Ph.D. Committee Member Assistant Professor Department of Chemistry University of Dayton ii © Copyright by Batool J. Murtadha All rights reserved 2020 iii ABSTRACT ASYMMETRIC SYNTHESIS OF ORGANOPHOSPHATES AND THEIR DERIVATIVES Name: Murtadha, Batool J. University of Dayton Advisor: Dr. Jeremy Erb Organophosphorus compounds (OPs) are widely used in the agricultural industry especially in the pesticide market. Phosphates play a huge role as biological compounds in the form of energy carrier compounds like ATP, and medicine as antivirals. OPs have become increasingly important as evidenced by the publication of new methods devoted to their uses and synthesis. These well-established studies lay the basis for industrial organic derivatives of phosphorus preparations. The current work explored methods of synthesizing chiral organophosphate triesters. We experimented with different processes roughly divided into either an electrophilic or nucleophilic strategy using chiral Lewis acids, organocatalysts (HyperBTM), activating agents, and chiral auxiliaries with the goal of control stereoselectivity. These methods were explored through the use of different starting materials like POCl3, triethyl phosphate, methyl phosphordichloradate, and PSCl3. -

Synthesis of 4-Phosphono Β-Lactams and Related Azaheterocyclic Phosphonates
SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES IR . KRISTOF MOONEN To Elza Vercauteren Promotor: Prof. dr. ir. C. Stevens Department of Organic Chemistry, Research Group SynBioC Members of the Examination Committee: Prof. dr. ir. N. De Pauw (Chairman) Prof. dr. J. Marchand-Brynaert Prof. dr. A. Haemers Prof. dr. S. Van Calenbergh Prof. dr. ir. E. Vandamme Prof. dr. ir. R. Verhé Prof. dr. ir. N. De Kimpe Dean: Prof. dr. ir. H. Van Langenhove Rector: Prof. dr. P. Van Cauwenberge IR . KRISTOF MOONEN SYNTHESIS OF 4-PHOSPHONO β-LACTAMS AND RELATED AZAHETEROCYCLIC PHOSPHONATES Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Chemistry Dutch translation of the title: Synthese van 4-fosfono-β-lactamen en aanverwante azaheterocyclische fosfonaten ISBN-Number: 90-5989-129-5 The author and the promotor give the authorisation to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. Woord Vooraf Toen ik op een hete dag in de voorbije zomer dit woord vooraf schreef, stond ik voor één van de laatste horden te nemen in de weg naar het “doctoraat”. Het ideale moment voor een nostalgische terugblik op een zeer fijne periode, hoewel het onzinnig zou zijn te beweren dat alles rozegeur en maneschijn was. En op het einde van de rit komt dan ook het moment waarop je eindelijk een aantal mensen kunt bedanken, omwille van sterk uiteenlopende redenen. -
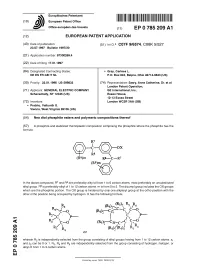
Neo Diol Phosphite Esters and Polymeric Compositions Thereof
^ ^ ^ ^ I ^ ^ ^ ^ ^ ^ II ^ II ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ I ^ European Patent Office Office europeen des brevets EP 0 785 209 A1 EUROPEAN PATENT APPLICATION (43) Date of publication: (51) |nt ci * C07F 9/6574, C08K 5/527 23.07.1997 Bulletin 1997/30 (21) Application number: 97300269.4 (22) Date of filing: 17.01.1997 (84) Designated Contracting States: • Gray, Carloss L. DE ES FR GB IT NL P.O. Box 843, Belpre, Ohio 45714-0843 (US) (30) Priority: 22.01.1996 US 589832 (74) Representative: Szary, Anne Catherine, Dr. et al London Patent Operation, (71) Applicant: GENERAL ELECTRIC COMPANY GE International, Inc., Schenectady, NY 12345 (US) Essex House, 12-13 Essex Street (72) Inventors: London WC2R 3AA (GB) • Prabhu, Vaikunth S. Vienna, West Virginia 26105 (US) (54) Neo diol phosphite esters and polymeric compositions thereof (57) A phosphite and stabilized themoplastic composition comprising the phosphite where the phosphite has the formula: In the above compound, R7 and R8 are preferably alkyl of from 1 to 6 carbon atoms, most preferably an unsubstituted alkyl group. R9 is preferably alkyl of 1 to 1 2 carbon atoms, m is from 0 to 5. The dicumyl group includes the OX groups which are the phosphite portion. The OX group is hindered by only one alkylaryl group at the ortho position with the other ortho position being occupied by hydrogen. X has the following formula: —r'-^C—C C— O ^c— o (Rsk C p- !/C p- *2 2 C— O (R5)r-CZ2-^c^ O) o (R5)/(R5)2' CM or lO 00 wherein R2 is independently selected from the consisting of alkyl having from 1 to 12 carbon atoms, Is- group groups z-, and z2 can be 0 or 1. -

OJC Vol 31(Special
ORIENTAL JOURNAL OF CHEMISTRY ISSN: 0970-020 X CODEN: OJCHEG An International Open Free Access, Peer Reviewed Research Journal 2015, Vol. 31, (Spl Edn): Month : Oct. www.orientjchem.org Pg. 01-12 Aminomethylenephosphonic Acids Syntheses and Applications (A Review) DIDIER VILLEMIN1 and MOHAMED AMINE DIDI2* 1Laboratoire de Chimie Moléculaire et Thioorganique, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ENSICAEN, 14050 Caen, France. 2Laboratory of Separation and Purification Technology, Tlemcen University, Faculty of Sciences, Department of Chemistry,Box 119, Algeria. *Corresponding author E-mail: [email protected] (Received: March 25, 2015; Accepted: May 10, 2015) http://dx.doi.org/10.13005/ojc/31.Special-Issue1.01 ABSTRACT A review. In this paper, were reviewed the aminomethylenephosphonic acids which have several synthesis routes and many applications as separation of metals and rare earth by solvant extraction, N-C-P in medicinal chemistry, pesticides/herbicides and water-decontamination systems, organometallic complexes: MOF, gas absorption, proton conductivity and Magnetic shielding tensors / aminotris(methylenephosphonates). Key words: Aminomethylphosphonic; solvent extraction; chelate; rare earth metals; pesticides; herbicides. INTRODUCTION O O H H N C N P Aminophosphonic acid can be an OH OH attractive molecule because it can be used in a OH large range of field. The aim of this report is amino acid aminophosphonic acid to summarize synthesis methods of Fig. 1: Similarity between amino acids aminomethylphosphonic acids and their and aminophosphonic acids applications. Most important characteristic of these This similarity gives to aminomethyl- compounds is their N-C-P molecular fragment that phosphonic acid biological applications in broadly defines them as analogues of amino acids, healthcare and agriculture.