SC-04-JM-04 Analysis of Jack Mackerel Otolith Microstucture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status and Prospects of Mackerel and Tuna Fishery in Bangladesh
Status and prospects of mackerel and tuna fishery in Bangladesh Item Type article Authors Rahman, M.J.; Zaher, M. Download date 27/09/2021 01:00:11 Link to Item http://hdl.handle.net/1834/34189 Bangladesh]. Fish. Res.) 10(1), 2006: 85-92 Status and prospects of mackerel and tuna fishery in Bangladesh M. J. Rahman* and M. Zaher1 Marine Fisheries & Technology Station, Bangladesh Fisheries Research Institute Cox's Bazar 4700, Bangladesh 1Present address: BFRI, Freshwater Station, Mvmensingh 2201, Bangladesh *Corresponding author Abstract Present status and future prospects of mackerel and tuna fisheries in Bangladesh were assessed during July 2003-June 2004. The work concentrated on the fishing gears, length of fishes, total landings and market price of the catch and highlighted the prospects of the fishery in Bangladesh. Four commercially important species of mackerels and tuna viz. Scomberomorus guttatus, Scomberomorus commerson, Rastrelliger kanagurta, and Euthynnus affinis were included in the study. About 95% of mackerels and tuna were caught by drift gill nets and the rest were caught by long lines ( 4%) and marine set-bag-net (1 %). Average monthly total landing of mackerels and tunas was about 264 t, of which 147 t landed in Cox's Bazar and 117 tin Chittagong sites. Total catches of the four species in Cox's Bazar and Chittagong sites were found to be 956 and 762 t, respectively. The poor landing was observed during January-February and the peak landing was in November and July. Gross market value of the annual landing of mackerels and tunas (1,718 t) was found to be 1,392 lakh taka. -

King Mackerel, Scomberomorus Caval/A, Mark-Recapture Studies Off Florida's East Coast
King Mackerel, Scomberomorus cavalla, Mark- Recapture Studies Off Florida's East Coast Item Type article Authors Schaefer, H. Charles; Fable, Jr. , William A. Download date 04/10/2021 08:14:11 Link to Item http://hdl.handle.net/1834/26474 King Mackerel, Scomberomorus caval/a, Mark-Recapture Studies Off Florida's East Coast H. CHARLES SCHAEFER and WILLIAM A. FABLE, JR. Introduction reproductive capacity causing stock re Panama City Laboratory, began a co ductions and declining recruitment operative mark-recapture study on king King mackerel, Scomberomorus cav (Godcharles I). King mackerel have mackerel to determine movements in alla, is a coastal, pelagic scombrid been jointly managed by the South At both the Gulf of Mexico and along the found off the U.S. Atlantic and Gulf of lantic and Gulf of Mexico Fishery Man Atlantic coast. Subsequently, biologists Mexico coasts. This species has histori agement Councils since the implemen from both agencies tagged king mack cally contributed to commercial and tation of the Coastal Pelagic Fishery erel (17,042 releases, 1,171 returns) recreational catches throughout its Management Plan (CPFMP) in 1983. from 1975 through 1979 (Sutherland range in the southeastern United States. The maximum sustainable yield (MSY) and Fable, 1980; Sutter et aI., 1991; Commercial exploitation intensified for the U.S. king mackerel resource is Williams and Godcharles3). Results there in the 1960's with the introduc currently estimated at 26.2 million from this study indicated that the spe tion of large power-assisted gillnet pounds (NMFS2). cies consisted of at least two migratory boats and spotter aircraft. -

Chub Mackerel, Scomber Japonicus (Perciformes: Scombridae), a New Host Record for Nerocila Phaiopleura (Isopoda: Cymothoidae)
生物圏科学 Biosphere Sci. 56:7-11 (2017) Chub mackerel, Scomber japonicus (Perciformes: Scombridae), a new host record for Nerocila phaiopleura (Isopoda: Cymothoidae) 1) 2) Kazuya NAGASAWA * and Hiroki NAKAO 1) Graduate School of Biosphere Science, Hiroshima University, 1-4-4 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8528, Japan 2) Fisheries Research Division, Oita Prefectural Agriculture, Forestry and Fisheries Research Center, Kamiura, Saeki, Oita 879-2602, Japan Abstract An ovigerous female of Nerocila phaiopleura Bleeker, 1857 was collected from the caudal peduncle of a chub mackerel, Scomber japonicus Houttuyn, 1782 (Perciformes: Scombridae), at the Hōyo Strait located between the western Seto Inland Sea and the Bungo Channell in western Japan. This represents a new host record for N. phaioplueura and its fourth record from the Seto Inland Sea and adjacent region. Key words: Cymothoidae, fish parasite, Isopoda, Nerocila phaiopleura, new host record, Scomber japonicus INTRODUCTION The Hōyo Strait is located between the western Seto Inland Sea and the Bungo Channell in western Japan. This strait is famous as a fishing ground of two perciform fishes of high quality, viz., chub mackerel, Scomber japonicus Houttuyn, 1782 (Scombridae), and Japanese jack mackerel, Trachurus japonicus (Temminck and Schlegel, 1844) (Carangidae), both of which are currently called“ Seki-saba” and“ Seki-aji”, respectively, as registered brands (e.g., Ishida and Fukushige, 2010). The brand names are well known nationwide, and the price of the fishes is very high (up to 5,000 yen per kg). Under these situations, the fishermen working in the strait pay much attention to the parasites of the fishes they catch because those fishes are almost exclusively eaten raw as“ sashimi.” Recently, a chub mackerel infected by a large parasite on the body surface (Fig. -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -
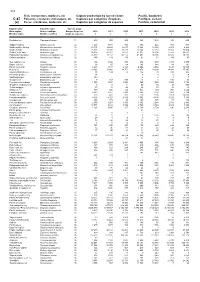
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Blue Jack Mackerel (Trachurus Picturatus) in Subdivision 10.A.2 (Azores Grounds)
ICES Advice on fishing opportunities, catch, and effort Bay of Biscay and the Iberian Coast ecoregion Published 18 December 2020 Blue jack mackerel (Trachurus picturatus) in Subdivision 10.a.2 (Azores grounds) ICES advice on fishing opportunities ICES advises that when the precautionary approach is applied, catches should be no more than 878 tonnes in each of the years 2021 and 2022. Note: This advice sheet is abbreviated due to the COVID-19 disruption. The previous advice issued for 2019 and 2020 is attached as Annex 1. Stock development over time Figure 1 Blue jack mackerel in Subdivision 10.a.2. Landings and other catches. Landings include purse-seine catches for human consumption – PS (HC) – purse-seine catches for bait – PS (Bait) – and include unsold purse-seine landings withdrawn at the port as well as longline and handline catches (LL and HL). Other catches include longline bait, tuna live bait, and recreational catches (incomplete in 2017–2019). Stock and exploitation status Table 1 Blue jack mackerel in Subdivision 10.a.2. State of the stock and fishery relative to reference points. ICES Advice 2020 – jaa.27..10a2 – https://doi.org/10.17895/ices.advice.7650 ICES advice, as adopted by its advisory committee (ACOM), is developed upon request by ICES clients (European Union, NASCO, NEAFC, Iceland, and Norway). 1 ICES Advice on fishing opportunities, catch, and effort Published 18 December 2020 jaa.27.10a2 Catch scenarios ICES framework for category 5 stocks was applied (ICES, 2012). For stocks without information on abundance or exploitation, ICES considers that a precautionary reduction of catches should be implemented unless there is ancillary information clearly indicating that the current level of exploitation is appropriate for the stock. -

Spanish Mackerel J
2.1.10.6 SSM CHAPTER 2.1.10.6 AUTHORS: LAST UPDATE: ATLANTIC SPANISH MACKEREL J. VALEIRAS and E. ABAD Sept. 2006 2.1.10.6 Description of Atlantic Spanish Mackerel (SSM) 1. Names 1.a Classification and taxonomy Species name: Scomberomorus maculatus (Mitchill 1815) ICCAT species code: SSM ICCAT names: Atlantic Spanish mackerel (English), Maquereau espagnol (French), Carita del Atlántico (Spanish) According to Collette and Nauen (1983), the Atlantic Spanish mackerel is classified as follows: • Phylum: Chordata • Subphylum: Vertebrata • Superclass: Gnathostomata • Class: Osteichthyes • Subclass: Actinopterygii • Order: Perciformes • Suborder: Scombroidei • Family: Scombridae 1.b Common names List of vernacular names used according to ICCAT, FAO and Fishbase (www.fishbase.org). The list is not exhaustive and some local names might not be included. Barbados: Spanish mackerel. Brazil: Sororoca. China: ᶷᩬ㤿㩪. Colombia: Sierra. Cuba: Sierra. Denmark: Plettet kongemakrel. Former USSR: Ispanskaya makrel, Korolevskaya pyatnistaya makrel, Pyatnistaya makrel. France: Thazard Atlantique, Thazard blanc. Germany: Gefleckte Königsmakrele. Guinea: Makréni. Italy: Sgombro macchiato. Martinique: Taza doré, Thazard tacheté du sud. Mexico: Carite, Pintada, Sierra, Sierra común. Poland: Makrela hiszpanska. Portugal: Serra-espanhola. Russian Federation: Ispanskaya makrel, Korolevskaya pyatnistaya makrel, Pyatnistaya makrel; ɦɚɤɪɟɥɶ ɢɫɩɚɧɫɤɚɹ. South Africa: Spaanse makriel, Spanish mackerel. Spain: Carita Atlántico. 241 ICCAT MANUAL, 1st Edition (January 2010) Sweden: Fläckig kungsmakrill. United Kingdom: Atlantic spanish mackerel. United States of America: Spanish mackerel. Venezuela: Carite, Sierra pintada. 2. Identification Figure 1. Drawing of an adult Atlantic Spanish mackerel (by A. López, ‘Tokio’). Characteristics of Scomberomorus maculatus (see Figure 1 and Figure 2) Atlantic Spanish mackerel is a small tuna species. Maximum size is 91 cm fork length and 5.8 kg weight (IGFA 2001). -

A Preliminary Global Assessment of the Status of Exploited Marine Fish and Invertebrate Populations
A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS June 30 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria. L.D. Palomares, Rainer Froese, Brittany Derrick, Simon-Luc Nöel, Gordon Tsui Jessika Woroniak Daniel Pauly A report prepared by the Sea Around Us for OCEANA June 30, 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria L.D. Palomares1, Rainer Froese2, Brittany Derrick1, Simon-Luc Nöel1, Gordon Tsui1, Jessika Woroniak1 and Daniel Pauly1 CITE AS: Palomares MLD, Froese R, Derrick B, Nöel S-L, Tsui G, Woroniak J, Pauly D (2018) A preliminary global assessment of the status of exploited marine fish and invertebrate populations. A report prepared by the Sea Around Us for OCEANA. The University of British Columbia, Vancouver, p. 64. 1 Sea Around Us, Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver BC V6T1Z4 Canada 2 Helmholtz Centre for Ocean Research GEOMAR, Düsternbrooker Weg 20, 24105 Kiel, Germany TABLE OF CONTENTS Executive Summary 1 Introduction 2 Material and Methods 3 − Reconstructed catches vs official catches 3 − Marine Ecoregions vs EEZs 3 − The CMSY method 5 Results and Discussion 7 − Stock summaries reports 9 − Problematic stocks and sources of bias 14 − Stocks in the countries where OCEANA operates 22 − Stock assessments on the Sea Around Us website 31 − The next steps 32 Acknowledgements 33 References 34 Appendices I. List of marine ecoregions by EEZ 37 II. Summaries of number of stock by region and 49 by continent III. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Age and Growth of King Mackerel, Scomberomorus Cavalla, from the U.S
Age and Growth of King Mackerel, Scomberomorus cavalla, From the U.S. Gulf of Mexico CHARLES S. MANOOCH, III, STEVEN P. NAUGHTON, CHURCHILL B. GRIMES, and LEE TRENT Introduction incomplete knowledge concerning recre resource management. Resulting data ational catch and effort. migratory pat may be used to evaluate the impacts of The importance of king mackerel, terns, stock identity, and large-scale life fishing on the stocks or determine how Scomberomorus cavalla. to recreational history studies. Fishermen, scientists, they respond to different levels and and commercial fisheries along the and fishery managers still recognize strategies of fishing. Most studies on the southeastern Atlantic and Gulf of Mexico these as priority research areas critical to age and growth of king mackerel have coasts of the United States has been thor the management of king mackerel stocks shared the deficiency of being restricted oughly documented (Manooch et aI., which are judged to be heavily exploited by either time or space (Manooch et al. 1978: Manooch, 1979: Collette and along both coasts. The National Marine 1978), however. Johnson et al. (1983) Russo. 1984). Unfortunately, the impor Fisheries Service. regional universities, provided the most comprehensive geo tance of this coastal migratory species and state conservation agencies have re graphic coverage. and the need for large-scale, regionally sponded to this need and have initiated Herein we report on an independent coordinated research has not been recog extensive research efforts under the study on the Gulf of Mexico king mack nized until recentlyl.2 Manooch et al. Marine Fisheries Initiative (MARFIN) erel management unit. The objectives (1978) provided an annotated bibliogra Program. -

2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Opportunities for Sustainable Fisheries in Japan
OPPORTUNITIES FOR SUSTAINABLE FISHERIES IN JAPAN O2 REPORT: OPPORTUNITIES FOR SUSTAINABLE FISHERIES IN JAPAN JANUARY 2016 THIS REPORT OFFERS PRACTICAL RECOMMENDATIONS TO HELP RESTORE FISHERIES AND COASTAL FISHING COMMUNITIES ACROSS THE JAPANESE ARCHIPELAGO © Ana Chang 2 CONTENT Introduction/Summary 4 State of Japanese Fisheries 5 The Japanese Seafood Supply Chain 8 Seafood Supply Chain - Upstream 8 Seafood Supply Chain - Downstream 9 Seafood Imports/Exports 11 Species in Focus: Tuna Supply Chain 12 Policy/Management 14 Sustainable Seafood in Japan 17 Survey of Japanese Consumers 17 Survey of Japanese Fishermen/Managers 18 Recommendations 19 References 21 Addendum: Rapid Assessments of Eleven Japanese Fisheries 3 Introduction/Summary If you want to witness a display of marine abundance and diversity unrivaled nearly anywhere on planet earth, don’t go to the Coral Triangle. Instead, head straight to the heart of Tokyo, grab your rubber boots and take a stroll through the cavernous Tsukiji fish market. From wild Kamchatka sockeye salmon to giant tuna from the Mediterranean to Maine lobster, Tsukiji sells it all in the largest seafood market in the world. The freshest and highest quality seafood in Tsukiji still comes from waters sur- rounding the Japanese archipelago, which hold some of the most productive fishing grounds on the planet. But domestic fisheries have been in decline for decades, due to overfishing, degraded ecosystems, and negative socio-economic factors. For the average Japanese consumer, this decline has caused higher prices at the market and increasing difficulties in enjoying traditional “washoku” food items. “Unagi” (eel), for example, went from a peak commercial catch of 232 metric tons in 1963 to a measly 5 tons by 2011.1 Meanwhile, the price quadrupled in the last decade alone.