Guidelines for the Management of Hereditary Colorectal Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes Daniel Herzig, M.D
CLINICAL PRACTICE GUIDELINES The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes Daniel Herzig, M.D. • Karin Hardimann, M.D. • Martin Weiser, M.D. • Nancy Yu, M.D. Ian Paquette, M.D. • Daniel L. Feingold, M.D. • Scott R. Steele, M.D. Prepared by the Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons he American Society of Colon and Rectal Surgeons METHODOLOGY (ASCRS) is dedicated to ensuring high-quality pa- tient care by advancing the science, prevention, and These guidelines are built on the last set of the ASCRS T Practice Parameters for the Identification and Testing of management of disorders and diseases of the colon, rectum, Patients at Risk for Dominantly Inherited Colorectal Can- and anus. The Clinical Practice Guidelines Committee is 1 composed of society members who are chosen because they cer published in 2003. An organized search of MEDLINE have demonstrated expertise in the specialty of colon and (1946 to December week 1, 2016) was performed from rectal surgery. This committee was created to lead interna- 1946 through week 4 of September 2016 (Fig. 1). Subject tional efforts in defining quality care for conditions related headings for “adenomatous polyposis coli” (4203 results) to the colon, rectum, and anus, in addition to the devel- and “intestinal polyposis” (445 results) were included, us- opment of Clinical Practice Guidelines based on the best ing focused search. The results were combined (4629 re- available evidence. These guidelines are inclusive and not sults) and limited to English language (3981 results), then prescriptive. -

Polyp Classification from the Microscope
11/12/2019 OUTLINE Serrated lesions: • Updates on terminology Polyp classification from the • Updates on sessile serrated polyposis microscope HGD / intramucosal carcinoma / carcinoma in situ: • Clarify the terminology David F. Schaeffer, MD, FRCPC • Discuss diagnostic fears of pathologists Assistant Professor, Department of Pathology and Laboratory Medicine, UBC The subtle polyp: Head, Department of Pathology and Laboratory Medicine, Vancouver Coastal Health Pathology Lead, BCCA Colon Cancer Screening Program • Are some polyps really only hidden from the pathologist? • Do additional levels help? 3 January 2018 October 2019 OUTLINE WHY CARE ABOUT SERRATED POLYPS? Serrated lesions: FIT not useful • Updates on terminology • Updates on sessile serrated polyposis HGD / intramucosal carcinoma / carcinoma in situ: • Clarify the terminology • Discuss diagnostic fears of pathologists The subtle polyp: At best moderate • Are some polyps really only hidden from the agreement between pathologist? expert GI pathologist • Do additional levels help? October 2019 October 2019 1 11/12/2019 SIMPLIFIED VIEW OF SERRATED PATHWAY TYPES OF SERRATED POLYPS UNTIL JULY 2019 65% 30% 5% Important points Hyperplastic polyp (HP) Sessile Serrated Traditional Serrated • SSPs probably develop from MVHPs: MVHPs aren’t completely Microvesicular (MVHP) adenoma/polyp (SSA/P) Adenoma (TSA) innocuous but transformation to SSP is likely a rare event (occurs Goblet cell rich (GCHP ) more commonly in the right colon) • Serrated pathway is characterized by hypermethylation of -

Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00375.x Published by Blackwell Publishing CME Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D. Foulkes, M.B., Ph.D.2 1Division of Gastroenterology, Department of Medicine, The Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, Quebec, Canada, and 2Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, Quebec, Canada Familial adenomatous polyposis (FAP) is an autosomal-dominant colorectal cancer syndrome, caused by a germline mutation in the adenomatous polyposis coli (APC) gene, on chromosome 5q21. It is characterized by hundreds of adenomatous colorectal polyps, with an almost inevitable progression to colorectal cancer at an average age of 35 to 40 yr. Associated features include upper gastrointestinal tract polyps, congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and other extracolonic malignancies. Gardner syndrome is more of a historical subdivision of FAP, characterized by osteomas, dental anomalies, epidermal cysts, and soft tissue tumors. Other specified variants include Turcot syndrome (associated with central nervous system malignancies) and hereditary desmoid disease. Several genotype–phenotype correlations have been observed. Attenuated FAP is a phenotypically distinct entity, presenting with fewer than 100 adenomas. Multiple colorectal adenomas can also be caused by mutations in the human MutY homologue (MYH) gene, in an autosomal recessive condition referred to as MYH associated polyposis (MAP). Endoscopic screening of FAP probands and relatives is advocated as early as the ages of 10–12 yr, with the objective of reducing the occurrence of colorectal cancer. -

3. Studies of Colorectal Cancer Screening
IARC HANDBOOKS COLORECTAL CANCER SCREENING VOLUME 17 This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Cancer-Preventive Strategies, which met in Lyon, 14–21 November 2017 LYON, FRANCE - 2019 IARC HANDBOOKS OF CANCER PREVENTION 3. STUDIES OF COLORECTAL CANCER SCREENING 3.1 Methodological considerations end-point of the RCT can be the incidence of the cancer of interest. Methods for colorectal cancer (CRC) screen- The observed effect of screening in RCTs is ing include endoscopic methods and stool-based dependent on, among other things, the partic- tests for blood. The two primary end-points for ipation in the intervention group and the limi- endoscopic CRC screening are (i) finding cancer tation of contamination of the control group. at an early stage (secondary prevention) and Low participation biases the estimate of effect (ii) finding and removing precancerous lesions towards its no-effect value, and therefore it must (adenomatous polyps), to reduce the incidence be evaluated and reported. Screening of controls of CRC (primary prevention). The primary by services outside of the RCT also dilutes the end-point for stool-based tests is finding cancer effect of screening on CRC incidence and/or at an early stage. Stool-based tests also have some mortality. If the screening modality being eval- ability to detect adenomatous polyps; therefore, a uated is widely used in clinical practice in secondary end-point of these tests is reducing the the region or regions where the RCT is being incidence of CRC. conducted, then contamination may be consid- erable, although it may be difficult and/or costly 3.1.1 Randomized controlled trials of to estimate its extent. -

An “Expressionistic” Look at Serrated Precancerous Colorectal Lesions Giancarlo Marra
Marra Diagnostic Pathology (2021) 16:4 https://doi.org/10.1186/s13000-020-01064-1 RESEARCH Open Access An “expressionistic” look at serrated precancerous colorectal lesions Giancarlo Marra Abstract Background: Approximately 60% of colorectal cancer (CRC) precursor lesions are the genuinely-dysplastic conventional adenomas (cADNs). The others include hyperplastic polyps (HPs), sessile serrated lesions (SSL), and traditional serrated adenomas (TSAs), subtypes of a class of lesions collectively referred to as “serrated.” Endoscopic and histologic differentiation between cADNs and serrated lesions, and between serrated lesion subtypes can be difficult. Methods: We used in situ hybridization to verify the expression patterns in CRC precursors of 21 RNA molecules that appear to be promising differentiation markers on the basis of previous RNA sequencing studies. Results: SSLs could be clearly differentiated from cADNs by the expression patterns of 9 of the 12 RNAs tested for this purpose (VSIG1, ANXA10, ACHE, SEMG1, AQP5, LINC00520, ZIC5/2, FOXD1, NKD1). Expression patterns of all 9 in HPs were similar to those in SSLs. Nine putatively HP-specific RNAs were also investigated, but none could be confirmed as such: most (e.g., HOXD13 and HOXB13), proved instead to be markers of the normal mucosa in the distal colon and rectum, where most HPs arise. TSAs displayed mixed staining patterns reflecting the presence of serrated and dysplastic glands in the same lesion. Conclusions: Using a robust in situ hybridization protocol, we identified promising tissue-staining markers that, if validated in larger series of lesions, could facilitate more precise histologic classification of CRC precursors and, consequently, more tailored clinical follow-up of their carriers. -

Effect Modification of Vitamin D Supplementation By
nutrients Article Effect Modification of Vitamin D Supplementation by Histopathological Characteristics on Survival of Patients with Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial Hideyuki Yonaga 1,2, Shinya Okada 3 , Taisuke Akutsu 1, Hironori Ohdaira 4, Yutaka Suzuki 4 and Mitsuyoshi Urashima 1,* 1 Division of Molecular Epidemiology, The Jikei University School of Medicine, 3–25–8, Nishi-Shimbashi, Minato-Ku, Tokyo 105-8461, Japan; [email protected] (H.Y.); [email protected] (T.A.) 2 Celgene K. K., JP TOWER 2–7–2 Marunouchi Chiyoda-ku, Tokyo 100-7010, Japan; [email protected] 3 Department of Pathology, International University of Health and Welfare Hospital, 537–3 Iguchi, Nasushiobara, Tochigi 329-2763, Japan; [email protected] 4 Department of Surgery, International University of Health and Welfare Hospital, 537–3 Iguchi, Nasushiobara, Tochigi 329-2763, Japan; [email protected] (H.O.); [email protected] (Y.S.) * Correspondence: [email protected]; Tel.: +81-3-3433-1111 (ext. 2405) Received: 13 September 2019; Accepted: 21 October 2019; Published: 22 October 2019 Abstract: Some coauthors of this study previously performed the AMATERASU randomized, double-blind, placebo-controlled trial of postoperative oral vitamin D supplementation (2000 IU/day) in 417 patients with stage I to III digestive tract cancer from the esophagus to the rectum who underwent curative surgery (UMIN000001977). We conducted a post-hoc analysis of the AMATERASU trial to explore the effects of modification of vitamin D supplementation by histopathological characteristics on survival. Among patients with poorly differentiated adenocarcinoma, the 5-year relapse-free survival rate of patients supplemented with vitamin D was 91% compared with 63% in the placebo group (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.08 to 0.78; P = 0.017; P for interaction = 0.023). -

Risk Factors Associated with Rectal Neuroendocrine Tumors: a Cross-Sectional Study
Author Manuscript Published OnlineFirst on May 9, 2014; DOI: 10.1158/1055-9965.EPI-14-0132 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Risk Factors Associated with Rectal Neuroendocrine Tumors: A Cross-Sectional Study Yoon Suk Jung1, Kyung Eun Yun2, Yoosoo Chang2,3, Seungho Ryu2,3, Jung Ho Park1, Hong Joo Kim1, Yong Kyun Cho1, Chong Il Sohn1, Woo Kyu Jeon1, Byung Ik Kim1, and Dong Il Park1 1Department of Internal Medicine, 2Center for Cohort Studies, Total Healthcare Center, 3Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine, Seoul, Korea Running title: Risk factors for rectal neuroendocrine tumors Corresponding author: Dong Il Park, MD, PhD Department of Internal Medicine Kangbuk Samsung Hospital Sungkyunkwan University School of Medicine 108, Pyung-Dong, Jongro-Ku, Seoul, Korea 110-746 Phone: +82-2-2001-2059, Fax: +82-2-2001-2049 E-mail: [email protected] Disclosure of Potential Conflicts of Interest: We have nothing to disclose. 1 Downloaded from cebp.aacrjournals.org on October 1, 2021. © 2014 American Association for Cancer Research. Author Manuscript Published OnlineFirst on May 9, 2014; DOI: 10.1158/1055-9965.EPI-14-0132 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Abstract Background: The incidence of rectal neuroendocrine tumors (NETs) has been increasing since the implementation of the screening colonoscopy. However, very little is known about risk factors associated with rectal NETs. We examined the prevalence of and the risk factors for rectal NETs in a Korean population. -

(ACRCSP) Post Polypectomy Surveillance Guidelines
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background ………………………………………………………………………………………... 3 Terms, Definitions and Practical Points……………………………………………………….. 4 Post Colonoscopy Screening……………………………………………………………………. 5 Post Polypectomy Surveillance…………………………………………………………………..6 References ………………………………………………………………………………………….12 ACRCSP Post Polypectomy Surveillance Guidelines - 3 Background Adherence to evidence based guidelines is supported by the reduction of interval colorectal cancers and colorectal cancer (CRC)- related mortality. Surveillance interval guidelines are based on the presumption that a high quality baseline colonoscopy was performed (i.e. that the colonoscopy was completed to the cecum, and that the colonic mucosa was well visualized). It is also important to ensure the completeness of polypectomy and that all polypectomy material was sent to pathology. Patients with a failed colonoscopy (for example due to inability to reach cecum or poor bowel preparation) should undergo repeat colonoscopy (either by same operator or referred, depending on the reason why the colonoscopy was incomplete) or, less preferably, diagnostic imaging of the colon by CT colonography. A system should be in place to ensure that all pathology reports are reviewed and that recommendations to primary care physician regarding surveillance intervals are adjusted as indicated. Endoscopists should make clear recommendations to primary care physicians about the need for and timing of subsequent colonoscopy. Considering that the recommendation largely depends on the histological findings, interval recommendation in patients with polyps should account for the pathology report instead of being made at the time of colonoscopy. The decision regarding surveillance interval should be based on the most advanced finding(s) at baseline colonoscopy. -

Location of Colorectal Adenomas and Serrated Polyps in Patients Under Age 50
International Journal of Colorectal Disease (2019) 34:2201–2204 https://doi.org/10.1007/s00384-019-03445-5 SHORT COMMUNICATION Location of colorectal adenomas and serrated polyps in patients under age 50 Zexian Chen1,2 & Jiancong Hu1,2 & Zheyu Zheng1,2 & Chao Wang3 & Dezheng Lin1 & Yan Huang3 & Ping Lan1,2 & Xiaosheng He1,2 Accepted: 25 October 2019 /Published online: 18 November 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background The incidence of colorectal cancer, especially located in distal colorectum, is rising markedly in young patients. Conventional adenomas and serrated polyps have been widely recognized as precursors of colorectal cancer. Aim To investigate the correlation of polyp feature with polyp location in patients under age 50. Method Patients under age 50 who had received colonoscopy were included from 2010 to 2018. Clinical data including number, location, size, and histopathology of polyps were collected. Odd ratios and 95% confidence interval of adenomas with their location were calculated. Result In total, 25,636 patients aged 18–49 were enrolled, among which 4485 patients had polyps, with polyp detection rate of 17.5%. A total of 2484 and 2387 patients had conventional adenomas and serrated polyps, respectively. 76.0% advanced adenomas and 69.5% ≥ 10-mm serrated polyps were located in the distal colorectum. The detection rate of advanced adenomas was higher in patients aged 45–49. Patients with adenomas especially advanced adenomas in the distal colorectum were more likely to have advanced adenoma in the proximal colon. Conclusion Among patients under age 50, advanced adenomas and ≥ 10-mm serrated polyps were predominantly in the distal colorectum. -
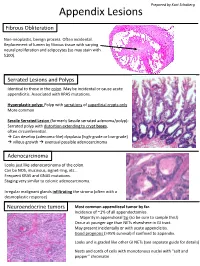
Appendix Lesions
Prepared by Kurt Schaberg Appendix Lesions Fibrous Obliteration Non-neoplastic, benign process. Often incidental. Replacement of lumen by fibrous tissue with varying neural proliferation and adipocytes (so may stain with S100). Serrated Lesions and Polyps Identical to those in the colon. May be incidental or cause acute appendicitis. Associated with KRAS mutations. Hyperplastic polyp: Polyp with serrations of superficial crypts only More common Sessile Serrated Lesion (formerly Sessile serrated adenoma/polyp): Serrated polyp with distortion extending to crypt bases, often circumferential. → Can develop (adenoma-like) dysplasia (high-grade or low-grade) → villous growth → eventual possible adenocarcinoma Adenocarcinoma Looks just like adenocarcinoma of the colon. Can be NOS, mucinous, signet-ring, etc… Frequent KRAS and GNAS mutations. Staging very similar to colonic adenocarcinoma. Irregular malignant glands infiltrating the stroma (often with a desmoplastic response) Neuroendocrine tumors Most common appendiceal tumor by far. Incidence of ~1% of all appendectomies. Majority in appendiceal tip (so be sure to sample this!) Occur at younger age than NETs elsewhere in GI tract. May present incidentally or with acute appendicitis. Good prognosis (>95% survival) if confined to appendix. Looks and is graded like other GI NETs (see separate guide for details) Nests and cords of cells with monotonous nuclei with “salt and pepper” chromatin Unique Appendiceal Lesions Appendiceal Mucinous Neoplasms Low-grade Appendiceal Mucinous Neoplasm (LAMN): -

Increased EZH2 Expression During the Adenoma‑Carcinoma Sequence in Colorectal Cancer
ONCOLOGY LETTERS 16: 5275-5281, 2018 Increased EZH2 expression during the adenoma‑carcinoma sequence in colorectal cancer MAYUKO OHUCHI1, YASUO SAKAMOTO1, RYUMA TOKUNAGA1, YUKI KIYOZUMI1, KENICHI NAKAMURA1, DAISUKE IZUMI1, KEISUKE KOSUMI1, KAZUTO HARADA1, JUNJI KURASHIGE1, MASAAKI IWATSUKI1, YOSHIFUMI BABA1, YUJI MIYAMOTO1, NAOYA YOSHIDA1, TAKASHI SHONO2, HIDEAKI NAOE2, YUTAKA SASAKI2 and HIDEO BABA1 Departments of 1Gastroenterological Surgery, and 2Gastroenterology and Hepatology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto 860-8556, Japan Received December 13, 2016; Accepted May 11, 2017 DOI: 10.3892/ol.2018.9240 Abstract. The adenoma-carcinoma sequence, the sequential from formalin‑fixed paraffin‑embedded blocks and reverse mutation and deletion of various genes by which colorectal transcription-quantitative polymerase chain reaction analysis cancer progresses, is a well-established and accepted concept of these genes was performed. Ki-67 and EZH2 expression of colorectal cancer carcinogenesis. Proteins of the polycomb scores increased significantly during the progression of repressive complex 2 (PRC2) function as transcriptional normal mucosa to adenoma and carcinoma (P=0.009), and repressors by trimethylating histone H3 at lysine 27; the EZH2 expression score was positively associated with Ki-67 activity of this complex is essential for cell proliferation expression score (P=0.02). Conversely, p21 mRNA and and differentiation. The histone methyltransferase enhancer protein expression decreased significantly, whereas expression of zeste homolog 2 (EZH2), an essential component of of p27 and p16 did not change significantly. During the PRC2, is associated with the transcriptional repression of carcinogenesis sequence from normal mucosa to adenoma and tumor suppressor genes. EZH2 expression has previously carcinoma, EZH2 expression increased and p21 expression been reported to increase with the progression of pancreatic decreased significantly. -

Copyrighted Material
Subject index Note: page numbers in italics refer to fi gures, those in bold refer to tables Abbreviations used in subentries abdominal mass patterns 781–782 GERD – gastroesophageal refl ux disease choledochal cysts 1850 perception 781 IBD – infl ammatory bowel disease mesenteric panniculitis 2208 periodicity 708–709 mesenteric tumors 2210 peripheral neurogenic 2429 A omental tumors 2210 pharmacological management 714–717 ABCB1/MDR1 484 , 633 , 634–636 abdominal migrane (AM) 712 , 2428–2429 physical examination 709–710 , 710 ABCB4 abdominal obesity 2230 postprandial 2498–2499 c h o l e s t e r o l g a l l s t o n e s 1817–1818 , abdominal pain 695–722 in pregnancy 842 1819–1820 a c u t e see acute abdominal pain prevalence 695 functions 483–484 , 1813 acute cholecystitis 784 rare/obscure causes 712–713 , 712 intrahepatic cholestasis of pregnancy 848 acute diverticulitis 794–796 , 795 , 1523–1524 , red fl ags 713 low phospholipid-associated 1527 relieving/aggravating factors 709 cholelithiasis 1810 , 1819–1820 , 2393 acute mesenteric ischemia 2492 right lower quadrant 794 m u t a t i o n p h e n o t y p e s 2392 , 2393 a c u t e p a n c r e a t i t i s 795 , 1653 , 1667 right upper quadrant 794 progressive familial intrahepatic cholestasis-3 acute suppurative peritonitis 2196 sickle cell crisis 2419 494 adhesions 713 s i t e 703 , 708 ABCB11 see bile salt export pump (BSEP) AIDS 2200 special pain syndromes 718–720 ABCC2/MRP2 485 , 494 , 870 , 1813 , 2394 , 2395 anxiety 706–707 sphincter of Oddi dysfunction 1877 ABCG2/BCRP 484–485 , 633