Colon Polyps Prepared by Kurt Schaberg
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juvenile Polyposis Syndrome Might Be
Gao et al. BMC Gastroenterology (2020) 20:167 https://doi.org/10.1186/s12876-020-01238-7 CASE REPORT Open Access Juvenile polyposis syndrome might be misdiagnosed as familial adenomatous polyposis: a case report and literature review Xian Hua Gao1,2†, Juan Li3†, Zi Ye Zhao1,2†, Xiao Dong Xu1,2,YiQiDu2,4, Hong Li Yan2,5, Lian Jie Liu1*, Chen Guang Bai2,6* and Wei Zhang1,2* Abstract Background: Juvenile polyposis syndrome (JPS) is a rare disorder characterized by the presence of multiple juvenile polyps in the gastrointestinal tract, and germline mutations in SMAD4 or BMPR1A. Due to its rarity and complex clinical manifestation, misdiagnosis often occurs in clinical practice. Case presentation: A 42-year-old man with multiple pedunculated colorectal polyps and concomitant rectal adenocarcinoma was admitted to our hospital. His mother had died of colon cancer. He was diagnosed with familial adenomatous polyposis (FAP) and underwent total proctocolectomy and ileal pouch anal anastomosis. Two polyps were selected for pathological examination. One polyp had cystically dilated glands with slight dysplasia. The other polyp displayed severe dysplasia and was diagnosed as adenoma. Three years later, his 21-year-old son underwent a colonoscopy that revealed more than 50 pedunculated colorectal juvenile polyps. Both patients harbored a germline pathogenic mutation in BMPR1A. Endoscopic resection of all polyps was attempted but failed. Finally, the son received endoscopic resection of polyps in the rectum and sigmoid colon, and laparoscopic subtotal colectomy. Ten polyps were selected for pathological examination. All were revealed to be typical juvenile polyps, with cystically dilated glands filled with mucus. -

Guidelines for the Management of Hereditary Colorectal Cancer
Guidelines Guidelines for the management of hereditary Gut: first published as 10.1136/gutjnl-2019-319915 on 28 November 2019. Downloaded from colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/ United Kingdom Cancer Genetics Group (UKCGG) Kevin J Monahan ,1,2 Nicola Bradshaw,3 Sunil Dolwani,4 Bianca Desouza,5 Malcolm G Dunlop,6 James E East,7,8 Mohammad Ilyas,9 Asha Kaur,10 Fiona Lalloo,11 Andrew Latchford,12 Matthew D Rutter ,13,14 Ian Tomlinson ,15,16 Huw J W Thomas,1,2 James Hill,11 Hereditary CRC guidelines eDelphi consensus group ► Additional material is ABSTRact having a family history of a first-degree relative published online only. To view Heritable factors account for approximately 35% of (FDR) or second degree relative (SDR) with CRC.2 please visit the journal online (http:// dx. doi. org/ 10. 1136/ colorectal cancer (CRC) risk, and almost 30% of the While highly penetrant syndromes such as Lynch gutjnl- 2019- 319915). population in the UK have a family history of CRC. syndrome (LS), familial adenomatous polyposis (FAP) The quantification of an individual’s lifetime risk of and other polyposis syndromes account for account For numbered affiliations see end of article. gastrointestinal cancer may incorporate clinical and for only 5–10% of all CRC diagnoses, advances molecular data, and depends on accurate phenotypic in genetic diagnosis, improvements in endoscopic Correspondence to assessment and genetic diagnosis. In turn this may surgical control, and medical and lifestyle interven- Dr Kevin J Monahan, Family facilitate targeted risk-reducing interventions, including tions provide opportunities for CRC prevention and Cancer Clinic, St Mark’s endoscopic surveillance, preventative surgery and effective treatment in susceptible individuals. -

Colonic Polyps in Children and Adolescents
durno_9650.qxd 26/03/2007 12:44 PM Page 233 INVITED REVIEW Colonic polyps in children and adolescents Carol A Durno MSc MD FRCPC CA Durno. Colonic polyps in children and adolescents. Can J Polypes du côlon chez les enfants et les Gastroenterol 2007;21(4):233-239. adolescents Colonic polyps most commonly present with rectal bleeding in chil- Les polypes du côlon se manifestent le plus fréquemment par des saigne- dren. The isolated juvenile polyp is the most frequent kind of polyp ments rectaux chez les enfants. Le polype juvénile isolé est le type de identified in children. ‘Juvenile’ refers to the histological type of polype le plus souvent observé chez les enfants. Précisons qu’ici, le terme polyp and not the age of onset of the polyp. Adolescents and adults « juvénile » fait référence au type histologique du polype et non à l’âge du with multiple juvenile polyps are at a significant risk of intestinal patient au moment de son développement. Les adolescents et les adultes cancer. The challenge for adult and pediatric gastroenterologists is qui présentent des polypes juvéniles multiples sont exposés à un risque determining the precise risk of colorectal cancer in patients with important de cancer de l’intestin. Le défi, pour les gastro-entérologues qui juvenile polyposis syndrome. Attenuated familial adenamatous poly- œuvrent auprès des adultes et des enfants est de déterminer le risque pré- posis (AFAP) can occur either by a mutation at the extreme ends of cis de cancer colorectal chez les patients atteints du syndrome de polypose the adenomatous polyposis coli gene or by biallelic mutations in the juvénile. -

Polyp Classification from the Microscope
11/12/2019 OUTLINE Serrated lesions: • Updates on terminology Polyp classification from the • Updates on sessile serrated polyposis microscope HGD / intramucosal carcinoma / carcinoma in situ: • Clarify the terminology David F. Schaeffer, MD, FRCPC • Discuss diagnostic fears of pathologists Assistant Professor, Department of Pathology and Laboratory Medicine, UBC The subtle polyp: Head, Department of Pathology and Laboratory Medicine, Vancouver Coastal Health Pathology Lead, BCCA Colon Cancer Screening Program • Are some polyps really only hidden from the pathologist? • Do additional levels help? 3 January 2018 October 2019 OUTLINE WHY CARE ABOUT SERRATED POLYPS? Serrated lesions: FIT not useful • Updates on terminology • Updates on sessile serrated polyposis HGD / intramucosal carcinoma / carcinoma in situ: • Clarify the terminology • Discuss diagnostic fears of pathologists The subtle polyp: At best moderate • Are some polyps really only hidden from the agreement between pathologist? expert GI pathologist • Do additional levels help? October 2019 October 2019 1 11/12/2019 SIMPLIFIED VIEW OF SERRATED PATHWAY TYPES OF SERRATED POLYPS UNTIL JULY 2019 65% 30% 5% Important points Hyperplastic polyp (HP) Sessile Serrated Traditional Serrated • SSPs probably develop from MVHPs: MVHPs aren’t completely Microvesicular (MVHP) adenoma/polyp (SSA/P) Adenoma (TSA) innocuous but transformation to SSP is likely a rare event (occurs Goblet cell rich (GCHP ) more commonly in the right colon) • Serrated pathway is characterized by hypermethylation of -

3. Studies of Colorectal Cancer Screening
IARC HANDBOOKS COLORECTAL CANCER SCREENING VOLUME 17 This publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Cancer-Preventive Strategies, which met in Lyon, 14–21 November 2017 LYON, FRANCE - 2019 IARC HANDBOOKS OF CANCER PREVENTION 3. STUDIES OF COLORECTAL CANCER SCREENING 3.1 Methodological considerations end-point of the RCT can be the incidence of the cancer of interest. Methods for colorectal cancer (CRC) screen- The observed effect of screening in RCTs is ing include endoscopic methods and stool-based dependent on, among other things, the partic- tests for blood. The two primary end-points for ipation in the intervention group and the limi- endoscopic CRC screening are (i) finding cancer tation of contamination of the control group. at an early stage (secondary prevention) and Low participation biases the estimate of effect (ii) finding and removing precancerous lesions towards its no-effect value, and therefore it must (adenomatous polyps), to reduce the incidence be evaluated and reported. Screening of controls of CRC (primary prevention). The primary by services outside of the RCT also dilutes the end-point for stool-based tests is finding cancer effect of screening on CRC incidence and/or at an early stage. Stool-based tests also have some mortality. If the screening modality being eval- ability to detect adenomatous polyps; therefore, a uated is widely used in clinical practice in secondary end-point of these tests is reducing the the region or regions where the RCT is being incidence of CRC. conducted, then contamination may be consid- erable, although it may be difficult and/or costly 3.1.1 Randomized controlled trials of to estimate its extent. -

An “Expressionistic” Look at Serrated Precancerous Colorectal Lesions Giancarlo Marra
Marra Diagnostic Pathology (2021) 16:4 https://doi.org/10.1186/s13000-020-01064-1 RESEARCH Open Access An “expressionistic” look at serrated precancerous colorectal lesions Giancarlo Marra Abstract Background: Approximately 60% of colorectal cancer (CRC) precursor lesions are the genuinely-dysplastic conventional adenomas (cADNs). The others include hyperplastic polyps (HPs), sessile serrated lesions (SSL), and traditional serrated adenomas (TSAs), subtypes of a class of lesions collectively referred to as “serrated.” Endoscopic and histologic differentiation between cADNs and serrated lesions, and between serrated lesion subtypes can be difficult. Methods: We used in situ hybridization to verify the expression patterns in CRC precursors of 21 RNA molecules that appear to be promising differentiation markers on the basis of previous RNA sequencing studies. Results: SSLs could be clearly differentiated from cADNs by the expression patterns of 9 of the 12 RNAs tested for this purpose (VSIG1, ANXA10, ACHE, SEMG1, AQP5, LINC00520, ZIC5/2, FOXD1, NKD1). Expression patterns of all 9 in HPs were similar to those in SSLs. Nine putatively HP-specific RNAs were also investigated, but none could be confirmed as such: most (e.g., HOXD13 and HOXB13), proved instead to be markers of the normal mucosa in the distal colon and rectum, where most HPs arise. TSAs displayed mixed staining patterns reflecting the presence of serrated and dysplastic glands in the same lesion. Conclusions: Using a robust in situ hybridization protocol, we identified promising tissue-staining markers that, if validated in larger series of lesions, could facilitate more precise histologic classification of CRC precursors and, consequently, more tailored clinical follow-up of their carriers. -

Huge Juvenile Polyps of the Stomach: a Case Report
Case Report Adv Res Gastroentero Hepatol Volume 6 Issue 3 - July 2017 DOI: 10.19080/ARGH.2017.06.555688 Copyright © All rights are reserved by Tsutomu Nishida Huge Juvenile Polyps of the Stomach: A Case Report Tsutomu Nishida1*, Hirotsugu Saiki1,2, Masashi Yamamoto1, Shiro Hayashi1, Tokuhiro Matsubara1, Sachiko Nakajima1, Masashi Hirota3, Hiroshi Imamura3, Ryoji Kushima4, Shiro Adachi5 and Masami Inada1 1Department of Gastroenterology, Toyonaka Municipal Hospital, Japan 2Department of Gastroenterology, Japan Community Health Care Organization Osaka Hospital, Japan 3Department of Surgery, Toyonaka Municipal Hospital, Japan 4Department of Clinical Laboratory Medicine, Shiga University of Medical Science, Japan 5Department of Pathology, Toyonaka Municipal Hospital, Japan Submission: July 10, 2017; Published: July 18, 2017 *Corresponding author: Tsutomu Nishida, Department of Gastroenterology, Toyonaka municipal Hospital, 4-14-1 Shibahara, Toyonaka, Osaka 560- 8565, Japan, Tel: ; Fax: ; Email: Abstract A 46-year-old man with no familial history of polyposis presented with diarrhea for 2 months. Laboratory data showed anemia, and mild hypoproteinemia. Computed tomography shows two huge tumors in the stomach. Esophagogastroduodenoscopy showed two huge polyps mucosa were partially reddish and had much mucin. All biopsy specimens from the polyps and randomly collected gastric mucosa indicated hyperplasticand giant folds changes. covering Colonoscopy nodular mucosa showed in theseveral stomach. sporadic Chromoendoscopy adenomatous polyps. with indigo We diagnosed carmine showedthe patient that with polyps huge with gastric finger-like hyperplastic villous polys causing protein losing and anemia and sporadic colonic adenomatous polyps. We performed gastrectomy. Immediately after surgery, he stopped diarrhea and recovered hemoglobin and serum protein levels. Histological examinations revealed that hyperplastic glands with cystically dilated glands were separated by abundant connective tissue. -

Hereditary Aspects of Colorectal Cancer Heather Hampel, MS, LGC the Ohio State University
Hereditary Aspects of Colorectal Cancer Heather Hampel, MS, LGC The Ohio State University Michael J. Hall, MD, MS Fox Chase Cancer Center Learning Objectives 1. Describe Lynch syndrome and identify patients at risk for having Lynch syndrome 2. Recognize other hereditary colorectal cancer syndromes, particularly polyposis conditions 3. Interpret immunohistochemical staining results for the four mismatch repair proteins and other tumor screening test results for Lynch syndrome 4. Understand the difference in cancer surveillance for individuals with Lynch syndrome compared to those in the general population 5. Describe the role of biomarkers (e.g., BRAF, KRAS, NRAS) and MSI-H in predicting response to targeted therapies used for the treatment of CRC CRC = colorectal cancer; MSI-H = microsatellite instability high. Financial Disclosure • Ms. Hampel is the PI of a grant that receives free genetic testing from Myriad Genetics Laboratories, Inc., is on the scientific advisory board for InVitae Genetics and Genome Medical, and has stock in Genome Medical. • Dr. Hall has nothing to disclose. Flowchart for Hereditary Colon Cancer Differential Diagnosis Presence of > 10 polyps Yes No Type of polyps Lynch syndrome Hamartomatous Adenomatous • Peutz-Jeghers syndrome • FAP • Juvenile polyposis • Attenuated FAP • Hereditary mixed polyposis • MUTYH-associated polyposis syndrome • Polymerase proofreading-associated • Serrated polyposis syndrome polyposis • Cowden syndrome FAP = familial adenomatous polyposis. Lynch Syndrome • Over 1.2 million individuals -
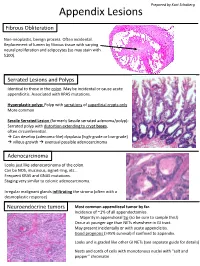
Appendix Lesions
Prepared by Kurt Schaberg Appendix Lesions Fibrous Obliteration Non-neoplastic, benign process. Often incidental. Replacement of lumen by fibrous tissue with varying neural proliferation and adipocytes (so may stain with S100). Serrated Lesions and Polyps Identical to those in the colon. May be incidental or cause acute appendicitis. Associated with KRAS mutations. Hyperplastic polyp: Polyp with serrations of superficial crypts only More common Sessile Serrated Lesion (formerly Sessile serrated adenoma/polyp): Serrated polyp with distortion extending to crypt bases, often circumferential. → Can develop (adenoma-like) dysplasia (high-grade or low-grade) → villous growth → eventual possible adenocarcinoma Adenocarcinoma Looks just like adenocarcinoma of the colon. Can be NOS, mucinous, signet-ring, etc… Frequent KRAS and GNAS mutations. Staging very similar to colonic adenocarcinoma. Irregular malignant glands infiltrating the stroma (often with a desmoplastic response) Neuroendocrine tumors Most common appendiceal tumor by far. Incidence of ~1% of all appendectomies. Majority in appendiceal tip (so be sure to sample this!) Occur at younger age than NETs elsewhere in GI tract. May present incidentally or with acute appendicitis. Good prognosis (>95% survival) if confined to appendix. Looks and is graded like other GI NETs (see separate guide for details) Nests and cords of cells with monotonous nuclei with “salt and pepper” chromatin Unique Appendiceal Lesions Appendiceal Mucinous Neoplasms Low-grade Appendiceal Mucinous Neoplasm (LAMN): -

Update on Polyp Surveillance Guidelines 2020
Update on Polyp Surveillance Guidelines 2020 Released 2020 health.govt.nz Citation: Ministry of Health. 2020. Update on Polyp Surveillance Guidelines. Wellington: Ministry of Health. Published in September 2020 by the Ministry of Health PO Box 5013, Wellington 6140, New Zealand ISBN 978-1-99-002937-0 (online) HP 7469 This document is available at health.govt.nz This work is licensed under the Creative Commons Attribution 4.0 International licence. In essence, you are free to: share ie, copy and redistribute the material in any medium or format; adapt ie, remix, transform and build upon the material. You must give appropriate credit, provide a link to the licence and indicate if changes were made. Contents Introduction 1 Purpose of this advice 3 Audience for these guidelines 3 Associated documents 4 Equity 4 Clinical advice 5 High-risk polyps 6 Notes on clinical context 6 Definitions 7 High-quality colonoscopy in New Zealand 7 Serrated polyposis syndrome 7 High-quality polypectomy 8 Multiple Adenoma referral criteria to the New Zealand Familial Gastrointestinal Cancer Service 8 Boston Bowel Preparation Scale 9 UPDATE ON POLYP SURVEILLANCE GUIDELINES iii Introduction Te Aho o Te Kahu (the Cancer Control Agency) in partnership with the National Screening Unit, Ministry of Health endorses this advice on surveillance colonoscopy for follow-up after removal of polyps. This advice sets out appropriate practice for clinicians to follow subject to their own judgement. It has been developed to help them make decisions in this area. The advice was developed to align with recent publications from the United Kingdom, the United States, Australia and Europe.1,2,3,4 These publications, based on updated available evidence up to June 2019, indicated that previous guidelines would now recommend over surveillance for some groups. -

Supplementary Endoscopy Report Form Colorectal Cancer Screening
Supplementary Endoscopy Report Form Colorectal Cancer Screening In Patients Treated With Radiation Therapy COLONOSCOPY FORM and PATHOLOGY REPORT Completed by:____________ Date: _____/_____/_____ MRN#: ________________________ Mo Day Year Participant Initials: ___________ Study Id No: ____________________ 1. Record the size, location, type of polyp, and procedures for removal of each polyp. Indicate location of polyp(s) on diagram below using assigned polyp letters. **Note: Assess size using open biopsy forcep** Location Shape Procedure Histology Atypia/Dysplasia CE = Cecum P = Peunculated 1 = Snare polypectomy C = Carcinoma H = High Grade Dysplasia AC = Ascending Colon S = Sessile 2 = Hot biopsy forceps N = Normal L = Low Grade Dysplasia HF = Hepatic Flexure U = Unable to be 3 = Cold biopsy H = Hyperplastic U = Unable to be TC = Transverse Colon determined, not 4 = Not removed T = Tubular determined, not mentioned SF = Splenic Flexure mentioned 5 = Lost/insufficient V = Villous N = None DC = Descending Colon M = Mixed Tubulovillous SC = Sigmoid Colon A = Adenomatous, not specified RE = Rectum U = Unable to be determined, not mentioned O = Other: ________________________________ Polyp Location Distance (cm) Diameter Shape Procedure Histology Atypia Letters from anal verge (mm) A _______ ___________ _______ _______ _______ _______ _______ B _______ ___________ _______ _______ _______ _______ _______ C _______ ___________ _______ _______ _______ _______ _______ D _______ ___________ _______ _______ _______ _______ _______ E _______ ___________ -

What's New in GI (PDF)
WHAT’S NEW IN PATHOLOGY? Issue 12 || February 2020 on “other tumors” covering mucosal • High grade appendiceal mucinous melanoma, germ cell tumors and neoplasm (HAMN) is added as a THE LATEST metastases. diagnostic entity similar to LAMN but showing high grade dysplasia. NEWS IN GI • Goblet cell carcinoid is renamed By Raul S. Gonzalez, M.D. Esophagus goblet cell adenocarcinoma, with • Gastroesophageal junction a new grading system. High grade examples represent what was he 5th Edition of the WHO Clas- carcinomas have been incorporated into the esophagus chapter. previously termed “adenocarcinoma Tsification of Tumours: Digestive ex goblet cell carcinoid” (Figure 1). System Tumours “blue book” was • Undifferentiated carcinoma is released in mid-2019. Key updates separated into its own entity rather are below. than a squamous cell carcinoma subtype. Lymphoepithelioma-like carcinoma is considered a subtype of General updates this entity. • The classification of digestive neuro- endocrine neoplasms is revised. They are categorized as neuroendocrine Stomach tumors (NETs, formerly “carcinoids”) • Undifferentiated carcinoma is now or neuroendocrine carcinomas its own separate entity with many (NECs) based primarily on histology. subtypes (large cell carcinoma with • NETs can be grade 1, 2 or 3, with rhabdoid phenotype, pleomorphic Figure 1: Goblet cell carcinoid of a Ki67 of less than 3% qualifying as the appendix is renamed goblet cell carcinoma, sarcomatoid carcinoma adenocarcinoma. grade 1 (formerly 0 - 2%). Mitotic and carcinoma with osteoclast-like rate is counted per 2 mm2, not per 10 giant cells). • Tubular carcinoid and L-cell high power fields. • Micropapillary adenocarcinoma carcinoid are renamed tubular NET • NECs are always high grade and is added as an aggressive subtype of and L-cell NET, respectively.