Winter Habitat Use by the Endangered, Migratory Swift Parrot (Lathamus Discolor) in New South Wales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Australia: Tasmania and the Orange- Bellied Parrot 24 – 29 October 2020 24 – 29 October 2021
AUSTRALIA: TASMANIA AND THE ORANGE- BELLIED PARROT 24 – 29 OCTOBER 2020 24 – 29 OCTOBER 2021 The handsome Orange-bellied Parrot is the primary target on this tour. www.birdingecotours.com [email protected] 2 | ITINERARY Australia: Tasmania and the Orange-bellied Parrot Adjoined to the mainland until the end of the last glacial period about ten thousand years ago, Tasmania is both geographically and genetically isolated from Australia. Through the millennia this island has developed its own unique set of plants and animals, including twelve avian endemics that include Forty-spotted Pardalote, Green Rosella, and Strong-billed Honeyeater. Beyond the endemics Tasmania also harbors several species which winter on the mainland and breed on Tasmania, such as Swift Parrot and Orange-bellied Parrot. These two breeding endemics are globally Critically Endangered (IUCN) and major targets on this tour. Forty-spotted Pardalote is one of the Tasmanian endemics we will target on this tour. Our search for the endemics and breeding specialties of Tasmania is set within a stunning backdrop of rugged coastlines, tall evergreen sclerophyll forests, alpine heathlands, and cool temperate rainforests, undoubtedly enriching our experience here. In addition, due to the lack of foxes many marsupials are notably more numerous in Tasmania, and we should be able to observe several of these unique animals during our stay. For those wishing to continue exploring Australia, this tour can be combined with our set of Australia tours: Australia: from the Outback to the Wet Tropics, Australia: Top End Birding, and Australia: Southwest Specialties. All four Australia tours could be combined. We can also arrange other extensions (e.g., sightseeing trips to Sydney, Uluru, etc., and pelagic trips). -

National Recovery Plan for the Swift Parrot (Lathamus Discolor)
National Recovery Plan for the Swift Parrot (Lathamus discolor) January 2019 The Species Profile and Threats Database pages linked to this recovery plan is obtainable from: http://www.environment.gov.au/cgi-bin/sprat/public/sprat.pl © Copyright Commonwealth of Australia, 2019. The National Recovery Plan for the Swift Parrot (Lathamus discolor) is licensed by the Commonwealth of Australia for use under a Creative Commons Attribution 4.0 International licence with the exception of the Coat of Arms of the Commonwealth of Australia, the logo of the agency responsible for publishing the report, content supplied by third parties, and any images depicting people. For licence conditions see: https://creativecommons.org/licenses/by/4.0/. This report should be attributed as ‘National Recovery Plan for the Swift Parrot (Lathamus discolor), Commonwealth of Australia 2019’. The Commonwealth of Australia has made all reasonable efforts to identify content supplied by third parties using the following format ‘© Copyright, [name of third party] ’. Disclaimer While reasonable efforts have been made to ensure that the contents of this publication are factually correct, the Commonwealth does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication. Image credits Front Cover: Swift Parrot. (© Copyright, Chris Tzaros). 2 Table of contents 1 Summary ..........................................................................................................4 -

Triplarina Nowraensis)
Draft NSW & National Recovery Plan Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) NSW NATIONAL Draft for Public Comment PARKS AND WILDLIFE March 2003 SERVICE © NSW National Parks and Wildlife Service, 2002. This work is copyright however, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NPWS. NSW National Parks and Wildlife Service 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 95856444 www.npws.nsw.gov.au Requests for information or comments regarding this recovery program are best directed to the Recovery Team via: The Nowra Heath Myrtle Recovery Team coordinator Threatened Species Unit, NPWS Southern Directorate PO Box 2115 Queanbeyan NSW 2620 Ph: (02) 6298 9700 email <[email protected]> Or The Director, Regional Wildlife Programs, Wildlife Australia Branch, Environment Australia, PO Box 636, Canberra ACT 2601 Ph: (02) 6274 1111 Cover illustration: Flowering branchlet of the Nowra Heath Myrtle Triplarina nowraensis. This plan should be cited as: NSW National Parks and Wildlife Service (2000) Draft NSW and National Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) NSW National Park and Wildlife Service, Hurstville NSW. ISBN: 0 7313 6192 X Draft Recovery Plan Nowra Heath-myrtle Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) Executive Summary This document constitutes the formal draft Commonwealth and New South Wales State Recovery Plan for the Nowra Heath-myrtle Triplarina nowraensis. -

Swift Parrot and Forty-Spotted Pardalote
Caption here. Swift Parrots are bright green and have red underwings and a long pointy tail. They get their name from their swift flight. a. Photo: Chris Tzaros SWIFT PARROTS NEED YOU TO LOOK AFTER YOUR BLUE AND BLACK GUMS c. Swift Parrots (Lathamus discolor) migrate each year from mainland Australia to Tasmania, breeding in late spring and summer when Blue gums and Black gums Nesting trees can be of any are flowering. sort of eucalypt as long as they have suitable hollows (a). Swift Parrots can only nest where there are old eucalypts Flowering Blue gum (b) and containing hollows, and where they can feed nearby on Black gum (c) trees are both the blossoms of Blue gum (Eucalyptus globulus) and important for foraging. Black gum (E. ovata). The more of these gums in the Photo: Rob Wiltshire area, and the closer they are to tree-hollows suitable for nesting, the better for the parrots. Swift Parrots have declined dramatically to fewer than 1,000 breeding pairs, chiefly because their forest habitat has b. been cleared and altered. All the main forest types used by breeding Swift Parrots have been affected in Tasmania; for instance, less than 30% of the original area of dry Blue gum forest remains, and only 3% of Black gum forest. Another significant threat is death or injury when their rapid flight causes them to collide with windows, fences and overhead wires. Changes to habitat can lead to increased competition from other birds which use tree-hollows for nesting in degraded areas. Because their survival is so tied to their breeding habitat, the fate of Swift Parrots is in our hands - every little action that we take today can go a long way towards ensuring that Photo: Chris Tzaros future generations continue to see and hear these birds. -

Corymbia Maculata) • Swamp Mahogany (E
Woodland Bird Identification and Survey Methods Workshop Welcome! Woo dlan d Bird Iden tifica tion and Survey MthdMethods WkhWorkshop BirdLife Australia’s Woodland Birds for Biodiversity project The aim of WBfB is to: Enhance the conservation of threatened and declining woodland birds in the temperate region of south-eastern Australia This project funded by: Also doing bird surveys at the tree- planting sites, including some that were planted in 1994 2 Workshop Summary 1. Getting to know woodland birds and their habitat - what are temperate woodlands and ‘woodland birds’? 2. Survey techniques for birds in temperate woodlands - getting started - woodland bird monitoring – the why’s and how’s - Regent Honeyeater habitat and search methods 3. Identification tips for birds in south-eastern Australia’s temperate woodlands - sight recognition - call recognition - useful “clues” - some tricky species - some important species Feel free to ask questions / initiate discussion as we go 3 1. What are temperate woodlands and ‘woodland birds’? What are temperate woodlands? • Woodlands are ecosystems with widely spaced trees (that seldom exceed 30m in height) whose crowns do not overlap • Open forests are often considered woodlands in Australia – and we do for the purposes of categorising “woodland birds” • Characterised by open understorey and sparse ground layer • Mainly on inland slopes of Great Divide / adjacent plains (e.g. Liverpool Plains) and drier, flatter areas in coastal catchments (e.g . Capertee / Hunter Valleys) • Almost all of temperate woodlands in south-east Australia are dominated by eucalypts, occasionally interspersed with native cypress pine, sheoak or buloke 5 Woodlands ain’t woodlands! • Numerous different types of woodlands exist • Most have a dominant species (e.g. -
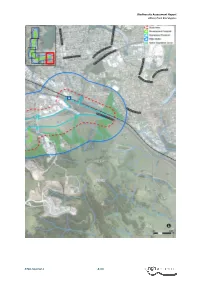
Biodiversity Offset Strategy ALBION PARK RAIL BYPASS
Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 B-VII Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX C CREDIT PROFILE As of 15/02/2017 Proposal ID for the assessment: 0035/2017/4182MP Version 1 (Calculator version 4) Assessment type: ‘Major Project’. 5726 Final V2.1 C-I Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-II Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-III Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-IV Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX D SEARS The project is considered State Significant Infrastructure and requires assessment under Part 5.1 of the EP&A Act. Biodiversity factors were assessed in an EIS, as per the Secretary Environmental Assessment Requirements (SEARs) for environmental impact assessment. The Final SEARs was provided by the Department of Planning and Environment on 18 March 2015. 5726 Final V2.1 D-I Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-II Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-III Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-IV Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-V Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VI Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VII Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VIII Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX E THREATENED SPECIES EVALUATIONS The following evaluation has been carried out for each listed entity of relevance to the project. -

Planting to Conserve Threatened Nomadic Pollinators in Nswdownload
Planting to conserve threatened nomadic pollinators in NSW Cover: Regent Honeyeater (Anthochaera Phrygia) - critically endangered species endemic to eastern Australia, Capertee Valley; Photo: Michael Todd © 2016 State of NSW and Office of Environment and Heritage The State of NSW and Office of Environment and Heritage are pleased to allow this material to be reproduced in whole or in part for educational and non-commercial use, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. The Office of Environment and Heritage (OEH) has compiled this statement in good faith, exercising all due care and attention. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. OEH shall not be liable for any damage which may occur to any person or organisation taking action or not on the basis of this publication. Readers should seek appropriate advice when applying the information to their specific needs. This document may be subject to revision without notice and readers should ensure they are using the latest version. Published by: Office of Environment and Heritage 59 Goulburn Street, Sydney NSW 2000 PO Box A290, Sydney South NSW 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131 555 (environmental information and publications requests) Phone: 1300 361 967 (national parks, general environmental inquiries and publications requests) Fax: (02) 9995 5999 TTY users: phone 133 677, then ask for 131 555 Speak and listen users: phone 1300 555 727, then ask for 131 555 Email: [email protected] Website: www.environment.nsw.gov.au Report pollution and environmental incidents Environment Line: 131 555 (NSW only) or [email protected] See also www.environment.nsw.gov.au ISBN 978-1-76039-469-1 OEH2016/0519 November 2016 Contents Acknowledgments ....................................................................................................... -

Swift Parrot (Lathamus Discolor)
Action Statement Flora and Fauna Guarantee Act 1988 No. 169 Swift Parrot Lathamus discolor Description and distribution The Swift Parrot Lathamus discolor Shaw 1790 (Psittaciformes: Platycercidae) is a non-breeding winter migrant to the mainland from Tasmania. It has a restricted breeding area in the east of Tasmania, arriving on the mainland in autumn to spend the winter period in foraging groups inhabiting forests and woodlands in south-east Australia. Swift Parrots are small to medium size birds reaching a length of 245mm, including a 120mm tail. Sexes are similar, except that the female is generally duller than the male. General plumage is green. Crown and ear coverts are dark blue and the face is red with yellow margins. Underparts are lighter and paler than back. Shoulder and underwing coverts are red, eye yellow and bill a horn colour. Brown (1989) and Higgins (1999) give Swift Parrot Latham discolor a full description of the species. (Photo: Mike Carter) Habitat In Victoria, the over-wintering habitat of the Swift Parrot is eucalypt forests and woodlands consisting primarily of the winter-flowering Grey Box (Eucalyptus microcarpa), Red Ironbark (Eucalyptus tricarpa), Mugga Ironbark (Eucalyptus sideroxylon) (far north-east Victoria), Yellow Gum (Eucalyptus leucoxylon) and White Box (Eucalyptus albens) (Brown 1989; Emison et al. 1987, C. Tzaros pers. comm.). They feed in gregarious flocks on nectar where eucalypts are in blossom or where lerps/psyllids are common. Blakers et al. (1985) describes Swift Parrots feeding on lerp psyllids amongst Red Gum (Eucalyptus camaldulensis) as Distribution in Victoria well as the aforementioned species. + before 1970, since 1970 [source: DSE 2004] In general terms, Grey Box flowers in autumn when the birds first arrive, followed by the Ironbarks with Yellow Gum and White Box often flowering Conservation status late prior to birds departing for Tasmania. -

Of Parrots 3 Other Major Groups of Parrots 16
ONE What are the Parrots and Where Did They Come From? The Evolutionary History of the Parrots CONTENTS The Marvelous Diversity of Parrots 3 Other Major Groups of Parrots 16 Reconstructing Evolutionary History 5 Box 1. Ancient DNA Reveals the Evolutionary Relationships of the Fossils, Bones, and Genes 5 Carolina Parakeet 19 The Evolution of Parrots 8 How and When the Parrots Diversified 25 Parrots’ Ancestors and Closest Some Parrot Enigmas 29 Relatives 8 What Is a Budgerigar? 29 The Most Primitive Parrot 13 How Have Different Body Shapes Evolved in The Most Basal Clade of Parrots 15 the Parrots? 32 THE MARVELOUS DIVERSITY OF PARROTS The parrots are one of the most marvelously diverse groups of birds in the world. They daz- zle the beholder with every color in the rainbow (figure 3). They range in size from tiny pygmy parrots weighing just over 10 grams to giant macaws weighing over a kilogram. They consume a wide variety of foods, including fruit, seeds, nectar, insects, and in a few cases, flesh. They produce large repertoires of sounds, ranging from grating squawks to cheery whistles to, more rarely, long melodious songs. They inhabit a broad array of habitats, from lowland tropical rainforest to high-altitude tundra to desert scrubland to urban jungle. They range over every continent but Antarctica, and inhabit some of the most far-flung islands on the planet. They include some of the most endangered species on Earth and some of the most rapidly expanding and aggressive invaders of human-altered landscapes. Increasingly, research into the lives of wild parrots is revealing that they exhibit a corresponding variety of mating systems, communication signals, social organizations, mental capacities, and life spans. -

Swift Parrot
Husbandry Guidelines for Photograph by Greg Miles, July 2011 Swift Parrot Lathamus discolor (Aves: Psittacidae) Date By From Version 2019 Crystal Capach RARES Fndn v 1 WORK HEALTH AND SAFETY RISKS Species Risk Category The Swift Parrot (Lathamus discolor) is considered a low risk, or innocuous parrot. The largest risk of harm would occur by the beak through biting, so care should be taken whilst handling or restraining the bird to reduce the chances of being bitten. WHS SUMMARY Though generally non aggressive in nature, injury may still occur through mishandling the bird, or through something as simple as working within the enclosure. A keeper must always take precautions whilst working to ensure their own safety and the safety of their fellow workers. If it happens that a serious injury occurs within the workplace, the regulator must be notified immediately in accordance with the Work Health and Safety Act 2011 (Workcover NSW). Workplace Risk Types 1. Biological There is a risk of zoonotic diseases being contracted when coming into contact with this species, as well as other bird species within the Psittacidae family. Namely, the highest zoonotic risk is the bacterial disease psittacosis, also known as ornithosis and chlamydiosis. Transmitted mainly through the inhalation of contaminated airborne faecal matter, feather dander, or beak-to-mouth contact, this disease can be diagnosed through the flu-like symptoms and respiratory tract illness presented. Maintaining proper hygiene practices and regular screening of birds should help prevent the zoonotic spread of this disease (Harkinezhad et al. 2009; West 2011). This disease as well as several others will be discussed further in Section 8. -

Swift Parrot (Lathamus Discolor)
Information Sheet NATIVE PLANTS AND ANIMALS THREATENED SPECIES AND COMMUNITIES OF THE ACT Swift Parrot (Lathamus discolor) A vulnerable species Issued: 4 March 2005 The Swift Parrot is a small, streamlined, bright grass- The species breeds only in Tasmania where it nests only green parrot, about 250 mm in length. It has a dusky red in hollows in mature and senescent eucalypts. It spike-shaped tail, red forehead and throat bordered by migrates north to mainland Australia over winter, yellow, and blue crown and cheeks. following abundances of flowering eucalypts and lerps as they occur. Small numbers of the species are recorded intermittently in the ACT. Grassy Blue Gum (E. globulus) forest in eastern Tasmania is the preferred habitat of the Swift Parrot in the breeding season. On the mainland, the species inhabits mainly dry open eucalypt forests and woodlands, usually box-ironbark communities and also Yellow Box–Red Gum woodland. Critical local habitat features include: I winter flowering eucalypts; and I eucalypts carrying lerps. In the ACT, the species is likely to occur anywhere below 700 m. ACT records of the species over the last 25 years are mainly from inner Canberra suburbs, Gungahlin and Hall. CONSERVATION THREATS Nationally, the range of the Swift Parrot has been reduced by half. The principal causes are loss of breeding and feeding habitat (Blue Gum Eucalyptus globulus, Messmate E. obliqua or Swamp Gum E. ovata The Swift Parrot is mainly an arboreal feeder, usually in forest) in Tasmania due to clearing for agriculture and the outer canopy of Eucalyptus trees. Their main food is urban development, and forestry operations.