Planting to Conserve Threatened Nomadic Pollinators in Nswdownload
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Printable PDF Format
Field Guides Tour Report Australia Part 2 2019 Oct 22, 2019 to Nov 11, 2019 John Coons & Doug Gochfeld For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Water is a precious resource in the Australian deserts, so watering holes like this one near Georgetown are incredible places for concentrating wildlife. Two of our most bird diverse excursions were on our mornings in this region. Photo by guide Doug Gochfeld. Australia. A voyage to the land of Oz is guaranteed to be filled with novelty and wonder, regardless of whether we’ve been to the country previously. This was true for our group this year, with everyone coming away awed and excited by any number of a litany of great experiences, whether they had already been in the country for three weeks or were beginning their Aussie journey in Darwin. Given the far-flung locales we visit, this itinerary often provides the full spectrum of weather, and this year that was true to the extreme. The drought which had gripped much of Australia for months on end was still in full effect upon our arrival at Darwin in the steamy Top End, and Georgetown was equally hot, though about as dry as Darwin was humid. The warmth persisted along the Queensland coast in Cairns, while weather on the Atherton Tablelands and at Lamington National Park was mild and quite pleasant, a prelude to the pendulum swinging the other way. During our final hours below O’Reilly’s, a system came through bringing with it strong winds (and a brush fire warning that unfortunately turned out all too prescient). -

Birds: Indicators of Environmental Repair in Oil Affected Coastlines
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Wells, Dezmond (2010) Birds : indicators of environmental repair in oil affected coastlines. (Unpublished) This file was downloaded from: https://eprints.qut.edu.au/59524/ c Copyright 2010 Dezmond Wells This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. Birds - Indicators of Environmental Repair in Oil Affected Coastlines 1 Birds - Indicators of Environmental Repair in Oil Affected Coastlines Dezmond. R. Wells (GradDipEd , BSc, , AssDipAppSc)A ABirds Australia Southern Queensland, 32 Panoramic Dr, Narangba, QLD 4504, Australia. Email: [email protected] Abstract Bird coastal communities were studied along Bribie Island and Moreton Island, two islands within Moreton Bay, Brisbane, Queensland, Australia, using the point counts method. -

Triplarina Nowraensis)
Draft NSW & National Recovery Plan Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) NSW NATIONAL Draft for Public Comment PARKS AND WILDLIFE March 2003 SERVICE © NSW National Parks and Wildlife Service, 2002. This work is copyright however, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NPWS. NSW National Parks and Wildlife Service 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 95856444 www.npws.nsw.gov.au Requests for information or comments regarding this recovery program are best directed to the Recovery Team via: The Nowra Heath Myrtle Recovery Team coordinator Threatened Species Unit, NPWS Southern Directorate PO Box 2115 Queanbeyan NSW 2620 Ph: (02) 6298 9700 email <[email protected]> Or The Director, Regional Wildlife Programs, Wildlife Australia Branch, Environment Australia, PO Box 636, Canberra ACT 2601 Ph: (02) 6274 1111 Cover illustration: Flowering branchlet of the Nowra Heath Myrtle Triplarina nowraensis. This plan should be cited as: NSW National Parks and Wildlife Service (2000) Draft NSW and National Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) NSW National Park and Wildlife Service, Hurstville NSW. ISBN: 0 7313 6192 X Draft Recovery Plan Nowra Heath-myrtle Recovery Plan for the Nowra Heath-myrtle (Triplarina nowraensis) Executive Summary This document constitutes the formal draft Commonwealth and New South Wales State Recovery Plan for the Nowra Heath-myrtle Triplarina nowraensis. -

Corymbia Maculata) • Swamp Mahogany (E
Woodland Bird Identification and Survey Methods Workshop Welcome! Woo dlan d Bird Iden tifica tion and Survey MthdMethods WkhWorkshop BirdLife Australia’s Woodland Birds for Biodiversity project The aim of WBfB is to: Enhance the conservation of threatened and declining woodland birds in the temperate region of south-eastern Australia This project funded by: Also doing bird surveys at the tree- planting sites, including some that were planted in 1994 2 Workshop Summary 1. Getting to know woodland birds and their habitat - what are temperate woodlands and ‘woodland birds’? 2. Survey techniques for birds in temperate woodlands - getting started - woodland bird monitoring – the why’s and how’s - Regent Honeyeater habitat and search methods 3. Identification tips for birds in south-eastern Australia’s temperate woodlands - sight recognition - call recognition - useful “clues” - some tricky species - some important species Feel free to ask questions / initiate discussion as we go 3 1. What are temperate woodlands and ‘woodland birds’? What are temperate woodlands? • Woodlands are ecosystems with widely spaced trees (that seldom exceed 30m in height) whose crowns do not overlap • Open forests are often considered woodlands in Australia – and we do for the purposes of categorising “woodland birds” • Characterised by open understorey and sparse ground layer • Mainly on inland slopes of Great Divide / adjacent plains (e.g. Liverpool Plains) and drier, flatter areas in coastal catchments (e.g . Capertee / Hunter Valleys) • Almost all of temperate woodlands in south-east Australia are dominated by eucalypts, occasionally interspersed with native cypress pine, sheoak or buloke 5 Woodlands ain’t woodlands! • Numerous different types of woodlands exist • Most have a dominant species (e.g. -

Eastern Australia: October-November 2016
Tropical Birding Trip Report Eastern Australia: October-November 2016 A Tropical Birding SET DEPARTURE tour EASTERN AUSTRALIA: From Top to Bottom 23rd October – 11th November 2016 The bird of the trip, the very impressive POWERFUL OWL Tour Leader: Laurie Ross All photos in this report were taken by Laurie Ross/Tropical Birding. 1 www.tropicalbirding.com +1-409-515-9110 [email protected] Page Tropical Birding Trip Report Eastern Australia: October-November 2016 INTRODUCTION The Eastern Australia Set Departure Tour introduces a huge amount of new birds and families to the majority of the group. We started the tour in Cairns in Far North Queensland, where we found ourselves surrounded by multiple habitats from the tidal mudflats of the Cairns Esplanade, the Great Barrier Reef and its sandy cays, lush lowland and highland rainforests of the Atherton Tablelands, and we even made it to the edge of the Outback near Mount Carbine; the next leg of the tour took us south to Southeast Queensland where we spent time in temperate rainforests and wet sclerophyll forests within Lamington National Park. The third, and my favorite leg, of the tour took us down to New South Wales, where we birded a huge variety of new habitats from coastal heathland to rocky shorelines and temperate rainforests in Royal National Park, to the mallee and brigalow of Inland New South Wales. The fourth and final leg of the tour saw us on the beautiful island state of Tasmania, where we found all 13 “Tassie” endemics. We had a huge list of highlights, from finding a roosting Lesser Sooty Owl in Malanda; to finding two roosting Powerful Owls near Brisbane; to having an Albert’s Lyrebird walk out in front of us at O Reilly’s; to seeing the rare and endangered Regent Honeyeaters in the Capertee Valley, and finding the endangered Swift Parrot on Bruny Island, in Tasmania. -
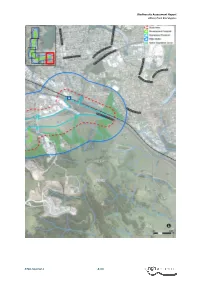
Biodiversity Offset Strategy ALBION PARK RAIL BYPASS
Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 B-VII Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX C CREDIT PROFILE As of 15/02/2017 Proposal ID for the assessment: 0035/2017/4182MP Version 1 (Calculator version 4) Assessment type: ‘Major Project’. 5726 Final V2.1 C-I Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-II Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-III Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 C-IV Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX D SEARS The project is considered State Significant Infrastructure and requires assessment under Part 5.1 of the EP&A Act. Biodiversity factors were assessed in an EIS, as per the Secretary Environmental Assessment Requirements (SEARs) for environmental impact assessment. The Final SEARs was provided by the Department of Planning and Environment on 18 March 2015. 5726 Final V2.1 D-I Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-II Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-III Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-IV Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-V Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VI Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VII Biodiversity Assessment Report Albion Park Rail Bypass 5726 Final V2.1 D-VIII Biodiversity Assessment Report Albion Park Rail Bypass APPENDIX E THREATENED SPECIES EVALUATIONS The following evaluation has been carried out for each listed entity of relevance to the project. -

Winter Habitat Use by the Endangered, Migratory Swift Parrot (Lathamus Discolor) in New South Wales
CSIRO PUBLISHING www.publish.csiro.au/journals/emu Emu, 2008, 108, 81–89 Winter habitat use by the endangered, migratory Swift Parrot (Lathamus discolor) in New South Wales Debra L. Saunders A,B and Robert HeinsohnA AThe Fenner School of Environment and Society, The Australian National University, Canberra, ACT 0200, Australia. BCorresponding author. Email: [email protected] Abstract. Migratory birds are dependent on a combination of suitable wintering, migration and breeding habitats. Identification and protection of these habitats is essential for the conservation of the birds. The endangered Swift Parrot (Psittacidae:Lathamus discolor) migrates north from Tasmania to south-eastern mainland Australia in search of suitable winter food resources. This 5-year study examines the use of known winter foraging habitats by Swift Parrots on a state- wide scale not previously attempted. Swift Parrots used a diversity of winter foraging habitats in regions of the coast and western slopes of New South Wales each year, including several habitats that occur in endangered ecological communi- ties. The abundance of Swift Parrots in New South Wales fluctuated significantly between years and regions, with coastal areas providing important drought-refuge habitats for a large proportion of the population. Over half of all foraging sites were used repeatedly, highlighting their likely importance for conservation. Landscapes containing winter foraging habitat included scattered trees, remnant vegetation and continuous forests, and Swift Parrots foraged extensively on lerp and nectar from a diversity of tree species within these. The occurrence of Swift Parrots at foraging sites was primarily asso- ciated with the abundance of lerp, nectar and non-aggressive competitors. -
OF the TOWNSVILLE REGION LAKE ROSS the Beautiful Lake Ross Stores Over 200,000 Megalitres of Water and Supplies up to 80% of Townsville’S Drinking Water
BIRDS OF THE TOWNSVILLE REGION LAKE ROSS The beautiful Lake Ross stores over 200,000 megalitres of water and supplies up to 80% of Townsville’s drinking water. The Ross River Dam wall stretches 8.3km across the Ross River floodplain, providing additional flood mitigation benefit to downstream communities. The Dam’s extensive shallow margins and fringing woodlands provide habitat for over 200 species of birds. At times, the number of Australian Pelicans, Black Swans, Eurasian Coots and Hardhead ducks can run into the thousands – a magic sight to behold. The Dam is also the breeding area for the White-bellied Sea-Eagle and the Osprey. The park around the Dam and the base of the spillway are ideal habitat for bush birds. The borrow pits across the road from the dam also support a wide variety of water birds for some months after each wet season. Lake Ross and the borrow pits are located at the end of Riverway Drive, about 14km past Thuringowa Central. Birds likely to be seen include: Australasian Darter, Little Pied Cormorant, Australian Pelican, White-faced Heron, Little Egret, Eastern Great Egret, Intermediate Egret, Australian White Ibis, Royal Spoonbill, Black Kite, White-bellied Sea-Eagle, Australian Bustard, Rainbow Lorikeet, Pale-headed Rosella, Blue-winged Kookaburra, Rainbow Bee-eater, Helmeted Friarbird, Yellow Honeyeater, Brown Honeyeater, Spangled Drongo, White-bellied Cuckoo-shrike, Pied Butcherbird, Great Bowerbird, Nutmeg Mannikin, Olive-backed Sunbird. White-faced Heron ROSS RIVER The Ross River winds its way through Townsville from Ross Dam to the mouth of the river near the Townsville Port. -

Review of Gene Movement by Bats and Birds and Its Potential Significance for Eucalypt Plantation Forestry
44 Gene movement by bats and birds Review of gene movement by bats and birds and its potential significance for eucalypt plantation forestry S.G. Southerton1,2, P. Birt3, J. Porter4 and H.A. Ford5 1CSIRO Division of Forestry and Forest Products, PO Box E4008, Kingston ACT 2600, Australia 2Email: [email protected] 3PO Box 108, Bellbowrie, Queensland 4070, Australia 43 Bougainvillea Ave, Indooroopilly, Queensland 4068, Australia 5Zoology, University of New England, Armidale, NSW 2351, Australia Revised manuscript received 30 May 2003 Summary Pollen-carrying vectors are the principal agents of gene movement in eucalypt forests. Eucalypt flowers produce abundant nectar Pollen- and/or nectar-feeding lorikeets and bats and nectar-feeding and pollen and hence attract numerous visitors, especially insects honeyeaters, while less frequent visitors to eucalypt flowers than and birds. Insects vastly outnumber all other floral visitors and insects, may make a unique contribution to eucalypt population undoubtedly play a major role in pollen movement (House 1997). structure because of their capacity to move pollen large distances. While birds and bats are less frequently recorded visitors to Birds and bats may travel upwards of 50 km day–1 during feeding, eucalypt flowers than insects, some species are dependent on and further during migration or feeding bouts over several days. flowers for food. These have the capacity to transport pollen over Limited data suggest that they carry viable pollen. Several much greater distances than insects. In this paper we examine the eucalypts have adaptations favouring bird pollinators, while some foraging behaviour, and feeding and migratory movements, of species, particularly Corymbia spp., have adaptations commonly birds and bats that feed on eucalypt flowers. -

A Preliminary Assessment of Faunal Values Within and Adjacent EPC 1029, Styx Basin, Central-East Queensland
A preliminary assessment of faunal values within and adjacent EPC 1029, Styx Basin, central-east Queensland ) Prepared for Yeats Consulting Engineers by Ed Meyer, Ecological Consultant,S Luscombe Street, Runcorn QLD 4113 ([email protected]) Conditions of use This report may only be used for the purposes for which it was commissioned. The use of this report, or part thereof, for any other reason or purpose is prohibited without the written consent of the author. Front cover: Fauna recorded from EPC 1029 during March 2011 surveys. Clockwise from upper left: ornamental snake (Denisonia maculata); squatter pigeon (southern race) (Geophaps scripta scripta); metallic snake-eyed skink (Cryptoblepharus metal/icus); and eastern sedgefrog (Litoria tal/ax). ©Edward Meyer 2011 5 Luscombe Street, Runcorn QLD 4113 E-mail:[email protected] Version 2 _ 3 August 2011 2 Table of contents 1. Summary 4 2. Background 6 Description of study area 6 Nomenclature 6 Abbreviations and acronyms 7 3. Methodology 9 General approach 9 ) Desktop assessment 9 Likelihood of occurrence assessments 10 Field surveys 11 Survey conditions 15 Survey limitations 15 4. Results 17 Desktop assessment findings 17 Likelihood of occurrence assessments 17 Field survey results -fauna 20 Field survey results - fauna habitat 22 Habitat for conservation significant species 28 ) 5. Summary and conclusions 37 6. References 38 Appendix A: Fauna previously recorded from Desktop Assessment Study Area 41 Appendix B: likelihood of occurrence assessments for conservation significant fauna 57 Appendix C: March 2011 survey results 73 Appendix D: Habitat photos 85 Appendix E: Habitat assessment proforma 100 3 1. Summary The faunal values of land within and adjacent Exploration Permit for Coal (EPe) 1029 were investigated by way of desktop review of existing information as well as field surveys carried out in late March 201l. -

Supplementary Material
10.1071/BT11174_AC Australian Journal of Botany 60(3), 165–199. CSIRO 2012 Supplementary Material Pollen morphology of the Myrtaceae. Part 1: tribes Eucalypteae, Lophostemoneae, Syncarpieae, Xanthostemoneae and subfamily Psiloxyloideae Andrew H. Thornhill, Geoff S. Hope, Lyn A. Craven and Michael D. Crisp Supplementary Table S1. List of taxa viewed with SEM ANBG - Australian Botanic Gardens, BRI - Queensland Herbarium, CANB, CBG - Australian National Herbarium, CSIRO, Canberra, DNA - Northern Territory Herbarium L - National Herbarium Nederland (Leiden), MI - University of Michigan Herbarium, NSW - National Herbarium of New South Wales, P - Herbier National de Paris. Taxon Voucher details Allosyncarpia ternata S.T.Blake C.R. Dunlop 4626, DNA Angophora costata Britten D. Nicolle 2103, CANB Angophora floribunda (Sm.) Sweet A. Gunnell 18 & W. Bishop, CANB Angophora hispida (Sm.) Blaxell Brooker 12948, CANB Angophora melanoxylon R.T.Baker leg. ign. 841, CANB Arillastrum gummiferum (Brongn. & Gris) Pancher ex Baill. G. McPherson 3580, CANB Corymbia maculata (Hook.) K.D.Hill & L.A.S.Johnson C.R. Dunlop s.n., CANB Corymbia variegata (F. Muell.) K.D.Hill & L.A.S.Johnson M.I.H. Brooker 3360, CANB (Accepted Corymbia citriodora (Hook.) K.D.Hill & L.A.S.Johnson) Eucalyptopsis papuana C.T.White M. Jacobs 9032, CANB Eucalyptus barklyensis L.A.S.Johnson & K.D.Hill K. Hill 3560 & L. Stanberg, DNA Eucalyptus cosmophylla F. Muell. M. Banks 1099, CANB Eucalyptus curtisii Blakely & C.T.White L.H. Bird s.n., NSW Eucalyptus globulus subsp. globulus Labill. T.A. Halliday 609, CANB Eucalyptus gunnii Hook.f. A. Moscal 14907, CANB Eucalyptus haemastoma Sm. R. Coveny 11354 & M. -

Reproductive Biology and Pollination in Rainforest Trees: Techniques for a Community-Level Approach BEST PRACTICE MANUAL
Reproductive Biology and Pollination in Rainforest Trees: Techniques for a Community-level Approach Techniques Reproductive Biology and Pollination in Rainforest Trees: BEST PRACTICE MANUAL Reproductive Biology and Pollination in Rainforest Trees: Techniques for a Community-level Approach S. L. Boulter, R. L. Kitching, J. M. Zalucki and K. L. Goodall Boulter et al. Rainforest CRC Headquarters at James Cook University, Smithfield, Cairns Postal address: PO Box 6811, Cairns, QLD 4870, AUSTRALIA Phone: (07) 4042 1246 Fax: (07) 4042 1247 Email: [email protected] http://www.rainforest-crc.jcu.edu.au The Cooperative Research Centre for Tropical Rainforest Ecology and Management (Rainforest CRC) is a research partnership involving the Cooperative Research Centre for Tropical Rainforest Ecology and Management Commonwealth and Queensland State Governments, the Wet Tropics Management Authority, the tourism industry, Aboriginal groups, the CSIRO, James Cook University, Griffith University and The University of Queensland. REPRODUCTIVE BIOLOGY AND POLLINATION IN RAINFOREST TREES: TECHNIQUES FOR A COMMUNITY-LEVEL APPROACH S. L. Boulter, R. L. Kitching, J. M. Zalucki and K. L. Goodall Australian School of Environmental Studies, Griffith University Established and supported under the Australian Cooperative Research Centres Program © Cooperative Research Centre for Tropical Rainforest Ecology and Management. ISBN 0 86443 763 3 This work is copyright. The Copyright Act 1968 permits fair dealing for study, research, news reporting, criticism or review. Selected passages, tables or diagrams may be reproduced for such purposes provided acknowledgment of the source is included. Major extracts of the entire document may not be reproduced by any process without written permission of the Chief Executive Officer, Cooperative Research Centre for Tropical Rainforest Ecology and Management.