To View Asset
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructure and Molecular Phylogenetic Position of a New Marine Sand-Dwelling Dinoflagellate from British Columbia, Canada: Pseudadenoides Polypyrenoides Sp
European Journal of Phycology ISSN: 0967-0262 (Print) 1469-4433 (Online) Journal homepage: http://www.tandfonline.com/loi/tejp20 Ultrastructure and molecular phylogenetic position of a new marine sand-dwelling dinoflagellate from British Columbia, Canada: Pseudadenoides polypyrenoides sp. nov. (Dinophyceae) Mona Hoppenrath, Naoji Yubuki, Rowena Stern & Brian S. Leander To cite this article: Mona Hoppenrath, Naoji Yubuki, Rowena Stern & Brian S. Leander (2017) Ultrastructure and molecular phylogenetic position of a new marine sand-dwelling dinoflagellate from British Columbia, Canada: Pseudadenoides polypyrenoides sp. nov. (Dinophyceae), European Journal of Phycology, 52:2, 208-224, DOI: 10.1080/09670262.2016.1274788 To link to this article: http://dx.doi.org/10.1080/09670262.2016.1274788 View supplementary material Published online: 03 Mar 2017. Submit your article to this journal Article views: 25 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tejp20 Download by: [The University of British Columbia] Date: 13 April 2017, At: 11:37 EUROPEAN JOURNAL OF PHYCOLOGY, 2017 VOL. 52, NO. 2, 208–224 http://dx.doi.org/10.1080/09670262.2016.1274788 Ultrastructure and molecular phylogenetic position of a new marine sand-dwelling dinoflagellate from British Columbia, Canada: Pseudadenoides polypyrenoides sp. nov. (Dinophyceae) Mona Hoppenratha,b, Naoji Yubukia,c, Rowena Sterna,d and Brian S. Leandera aDepartments of Botany and Zoology, -

Presencia Del Género Karenia Y Nuevos Registros De Dinoflagelados (Dinoflagellata) En Aguas De Las Islas Canarias, Atlántico Centro-Oriental
INFORME TÉCNICO nº15 INSTITUTO CANARIO DE CIENCIAS MARINAS Presencia del género Karenia y nuevos registros de dinoflagelados (Dinoflagellata) en aguas de las Islas Canarias, Atlántico Centro-Oriental - . '1 :=~:::::::::::= ~" Alicia Ojeda Créditos Edita: Instituto Canario de Ciencias Marinas Agencia Canaria de Investigación, Innovación y Sociedad de la Información Gobierno de Canarias Autora: Alicia Ojeda Rodríguez Diseño y maquetación: BlaBla Comunicación • www.blablacomunicacion.com Copyright © 2013 Alicia Ojeda Rodríguez - Instituto Canario de Ciencias Marinas (Gobierno de Canarias) Reservados todos los derechos. Queda rigurosamente prohibida la reproducción total o parcial de esta obra por cualquier medio o procedimiento, sin el permiso expreso y por escrito de los titulares de los derechos. Editado en febrero de 2013. Depósito Legal: GC 464-2013 ISSN: 1136-193X INFORME TÉCNICO nº15 INSTITUTO CANARIO DE CIENCIAS MARINAS Presencia del género Karenia y nuevos registros de dinoflagelados (Dinoflagellata) en aguas de las Islas Canarias, Atlántico Centro-Oriental Alicia Ojeda Presencia del género Karenia y nuevos registros de dinoflagelados (Dinoflagellata) en aguas de las Islas Canarias, Atlántico Centro-Oriental RESUMEN Se ilustran y describen 27 especies de dinoflagelados observados por primera vez en aguas superficiales neríticas y oceánicas de las Islas Canarias, Atlántico centro-oriental. Las especies descritas de Karenia, Ostreopsis y Prorocentrum revisten especial interés debido a su capacidad para sintetizar fuertes toxinas que durante periodos de floraciones algales pueden ocasionar importantes daños a la fauna marina y a la salud pública. Palabras clave: Dinoflagelados, Karenia, Ostreopsis, Prorocentrum, Islas Canarias, Atlántico centro-oriental. ABSTRACT 27 species of dinoflagellates from neritic and oceanic waters of the Canary Islands, Central- Eastern Atlantic Ocean are illustrated and described. -

Brachidiniales, Dinophyceae) in the Open Mediterranean Sea
Color profile: Disabled Composite 150 lpi at 45 degrees Acta Bot. Croat. 70 (2), 209–214, 2011 CODEN: ABCRA25 ISSN 0365-0588 eISSN 1847-8476 DOI: 10.2478/v10184-010-0019-0 Diversity and distribution of the dinoflagellates Brachidinium, Asterodinium and Microceratium (Brachidiniales, Dinophyceae) in the open Mediterranean Sea FERNANDO GÓMEZ* Instituto Cavanilles de Biodiversidad y Biología Evolutiva, Universidad de Valencia, PO Box 22085, 46071 Valencia, Spain Abstract – Brachidiniacean dinoflagellates have been investigated in the open waters of the Mediterranean Sea, along a transect from the south of France to the south of Cyprus (20 June–18 July 2008). Brachidinium and Karenia papilionacea often co-occurred, B. capitatum predominating in the surface waters. The highest abundance of Brachidinium were found in the upper 25 m in the western Mediterranean with a maximum (24 cells L–1) atadepthof5mintheBalearic Sea. Asterodinium (up to 4 cells L–1) was recorded below of deep chlorophyll maxima. The genus Microceratium, only known from the tropical Indo-Pacific region, is reported for the first time in the Mediterranean Sea. Microceratium was found below 100 m in the eastern Mediterranean Sea, with the highest abundance of 8 cells L–1 at 125 m depth, in the Levantine Basin. This study also illustrates for the first time specimens under the division of Brachidinium and Microceratium. This first occur- rence of Microceratium in the Mediterranean Sea should be considered an indicator of cli- mate warming. However, it should not be considered a non-indigenous taxon. Micro- ceratium is the 'tropical morphotype', the adaptation of a local species (a life stage of Karenia – Brachidinium – Asterodinium) to the tropical environmental conditions that prevail in summer in the open Mediterranean Sea. -

Morphological Studies of the Dinoflagellate Karenia Papilionacea in Culture
MORPHOLOGICAL STUDIES OF THE DINOFLAGELLATE KARENIA PAPILIONACEA IN CULTURE Michelle R. Stuart A Thesis Submitted to the University of North Carolina Wilmington in Partial Fulfillment of the Requirements for the Degree of Master of Science Department of Biology and Marine Biology University of North Carolina Wilmington 2011 Approved by Advisory Committee Alison R. Taylor Richard M. Dillaman Carmelo R. Tomas Chair Accepted by __________________________ Dean, Graduate School This thesis has been prepared in the style and format consistent with the journal Journal of Phycology ii TABLE OF CONTENTS ABSTRACT ................................................................................................................................... iv ACKNOWLEDGMENTS .............................................................................................................. v DEDICATION ............................................................................................................................... vi LIST OF TABLES ........................................................................................................................ vii LIST OF FIGURES ..................................................................................................................... viii INTRODUCTION .......................................................................................................................... 1 MATERIALS AND METHODS .................................................................................................... 5 RESULTS -

Self-Study 2008-2015 Oceanography, College of Geosciences Texas A&M University Self-Study 2008-2015
Self-Study 2008-2015 Oceanography, College of Geosciences Texas A&M University Self-Study 2008-2015 Executive Summary Welcome from the Department Chapter 1. Introduction to TAMU and TAMU Oceanography Chapter 2. The Mission and Goals of TAMU Oceanography Chapter 3. Evolution of the Department of Oceanography Since 2008 Chapter 4. Departmental Structure and Personnel Chapter 5. Department Resources Chapter 6. Contributions to University Research and Educational Excellence Chapter 7. Academic Programs and Curricula Chapter 8. Student Profile and Contributions Chapter 9. Concluding Observations Appendices Appendix 1: May 2014 Strategic Plan Appendix 2: Faculty Curriculum Vitae Appendix 3: Grants Awarded to the Ad-loc Faculty Appendix 4: Publications listed by year for the Ad-loc Faculty Appendix 5: Oceanography Courses Executive Summary The Department of Oceanography at Texas A&M University, founded in 1949 as the first oceanographic department established at an academic institution, is deeply committed to the unique mission of an AAU member institution with Land-, Sea-, and Space- grant status. With strengthened ties to other marine related units at the College Station and Galveston campuses, and significant investments from the University, we are poised to realize our strategic vision to join the nation’s top rank of institutions for oceanographic research and education at public universities. As part of our 2014 strategic planning effort, we have restructured our departmental educational, research and engagement activities into four interdisciplinary areas of strength: Ocean Observing Science and Technology, Marine Ecosystems Science and Health, Ocean Climate, and Ocean Energy. Observations, in a very broad sense, form the baseline for all of our strategic interdisciplinary themes, and are at the core of our vision to transform STEM education through a focus on big data competency. -

Is Karenia a Synonym of Asterodinium-Brachidinium (Gymnodiniales, Dinophyceae)?
Acta Bot. Croat. 64 (2), 263–274, 2005 CODEN: ABCRA25 ISSN 0365–0588 Is Karenia a synonym of Asterodinium-Brachidinium (Gymnodiniales, Dinophyceae)? FERNANDO GÓMEZ1*, YUKIO NAGAHAMA2,HARUYOSHI TAKAYAMA3,KEN FURUYA2 1 Station Marine de Wimereux, Université des Sciences et Technologies de Lille, CNRS UMR 8013 ELICO, 28 avenue Foch, BP 80, F-62930 Wimereux, France. 2 Department of Aquatic Biosciences, University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657, Japan. 3 Hiroshima Prefectural Fisheries and Marine Technology Center, Hatami 6-1-21, Ondo-cho, Kure Hiroshima 737-1205, Japan From material collected in open waters of the NW and Equatorial Pacific Ocean the de- tailed morphology of brachidiniaceans based on two specimens of Asterodinium gracile is reported for the first time. SEM observations showed that the straight apical groove, the morphological characters and orientation of the cell body were similar to those described for species of Karenia. Brachidinium and Asterodinium showed high morphological vari- ability in the length of the extensions and intermediate specimens with Karenia. Karenia-like cells that strongly resemble Brachidinium and Asterodinium but lacking the extensions co-occurred with the typical specimens. The life cycle and morphology of Karenia papilionacea should be investigated under natural conditions because of the strong simi- larity with the brachidiniaceans. Key words: Phytoplankton, Asterodinium, Brachidinium, Brachydinium, Gymnodinium, Karenia, Dinophyta, apical groove, SEM, Pacific Ocean. Introduction Fixatives, such as formaline or Lugol, do not sufficiently preserve unarmoured dino- flagellates to allow species identification. Body shape and morphology often change dur- ing the process of fixation so that even differentiating between the genera Gymnodinium Stein and Gyrodinium Kofoid et Swezy is difficult (ELBRÄCHTER 1979). -
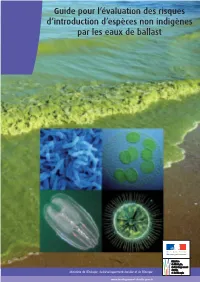
Guide Pour L'évaluation Des Risques D'introduction D'espèces Non
Guide pour l’évaluation des risques d’introduction d’espèces non indigènes par les eaux de ballast Contacts : Océane RIGNAULT & Damien CHEVALLIER Ministère de l’Écologie, du Développement durable et de l’Énergie Direction générale des Infrastructures, des Transports et de la Mer Direction des Affaires maritimes/Sous-direction de la sécurité maritime Téléphone : 01 40 81 21 22 © Credit photo (couverture) : photo de fond : Blue-green algae at beach, Toxic algae bloom on Lake Erie in 2011 Tom Archer, handout Les quatres petites photos : Scanning electron microscope image of Vibrio cholerae bacteria, which infect the digestive system.Zeiss DSM 962 SEM ; Mnemiopsis leidyi (in the central Baltic Sea in January 2008), Jan-Erik Bruun, FIMR (Finnish Institute of Marine Research) ; Cyanobacteria, Josef Reischig / CC BY-SA 3.0 ; Lake-bottom-blanketing zebra mussel, Maria Antónia Sampayo. Remerciements aux contributeurs : Perrine Prigent, Chargée de missions à la Direction des affaires maritimes, Laurent Guérin pour le Muséum national d’histoire naturelle, Daniel Masson pour l’IFREMER, Frédéric Quemmerais pour l’Agence des aires marines protégées, la Direction de l’eau et de la biodiversité, la Direction des services de transports. Ministère de l’Écologie, du Développement durable et de l’Énergie Direction générale des Infrastructures, des Transports et de la Mer Direction des affaires maritimes Tour Séquoia - 92055 La Défense cedex Tél. : 01 40 81 21 22 européen écolabel certifié papier du sur Imprimé SG/SPSSI/ATL2. : Impression - Cudelou SG/SPSSI/ATL2/Benoît -

(DOP) in the Dinoflagellate Peridinium Bipes by Physiological And
Yang et al. Environ Sci Eur (2020) 32:38 https://doi.org/10.1186/s12302-020-00317-6 RESEARCH Open Access Unveiling the impact of glycerol phosphate (DOP) in the dinofagellate Peridinium bipes by physiological and transcriptomic analysis Yanjun Yang1, Junqiong Shi1, Yunlu Jia2, Fang Bai2, Songqi Yang1, Wenmei Mi1, Shuhan He1 and Zhongxing Wu1* Abstract Background: The ability to use dissolved organic phosphorus (DOP) is important for survival and competition when phytoplankton are faced with scarcity of dissolved inorganic phosphorus (DIP). However, phosphorus availability to the freshwater dinofagellate Peridinium bipes has received relatively little attention, the efciency of glycerol phos- phate use by phytoplankton has rarely been investigated, and the regulatory molecular mechanisms remain unclear. Result: In the present study, cultures of the freshwater dinofagellate Peridinium bipes were set up in 119 medium ( DIP), DIP-depleted 119 medium (P-free), and β-glycerol phosphate-replacing-DIP medium ( DOP). Gene expres- sion+ was analyzed using transcriptomic sequencing. The growth rate of cells in DOP treatment+ group was similar to that in DIP group, but chlorophyll a fuorescence parameters RC/CS0, ABS/CS0, TR0/CS0, ET0/CS0 and RE0/CS0 markedly decreased in the DOP group. Transcriptomic analysis revealed that genes involved in photosynthesis, including psbA, psbB, psbC, psbD, psaA and psaB, were downregulated in the DOP group relative to the DIP group. Glycerol-3-phos- phate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase, rather than alkaline phosphatase, were responsible for β-glycerol phosphate use. Intercellular gluconeogenesis metabolism was markedly changed in the DOP group. In addition, genes involved in ATP synthases, the TCA cycle, oxidative phosphorylation, fatty acid metabo- lism and amino acid metabolism in P. -

Non-Native Marine Species in the Channel Islands: a Review and Assessment
Non-native Marine Species in the Channel Islands - A Review and Assessment - Department of the Environment - 2017 - Non-native Marine Species in the Channel Islands: A Review and Assessment Copyright (C) 2017 States of Jersey Copyright (C) 2017 images and illustrations as credited All rights reserved. No part of this report may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission of the States of Jersey. A printed paperback copy of this report has been commercially published by the Société Jersiaise (ISBN 978 0 901897 13 8). To obtain a copy contact the Société Jersiaise or order via high street and online bookshops. Contents Preface 7 1 - Background 1.1 - Non-native Species: A Definition 11 1.2 - Methods of Introduction 12 1.4 - Threats Posed by Non-Native Species 17 1.5 - Management and Legislation 19 2 – Survey Area and Methodology 2.1 - Survey Area 23 2.2 - Information Sources: Channel Islands 26 2.3 - Information Sources: Regional 28 2.4 –Threat Assessment 29 3 - Results and Discussion 3.1 - Taxonomic Diversity 33 3.2 - Habitat Preference 36 3.3 – Date of First Observation 40 3.4 – Region of Origin 42 3.5 – Transport Vectors 44 3.6 - Threat Scores and Horizon Scanning 46 4 - Marine Non-native Animal Species 51 5 - Marine Non-native Plant Species 146 3 6 - Summary and Recommendations 6.1 - Hotspots and Hubs 199 6.2 - Data Coordination and Dissemination 201 6.3 - Monitoring and Reporting 202 6.4 - Economic, Social and Environmental Impact 204 6.5 - Conclusion 206 7 - -

Brevetoxin-Producing Spherical Cells Present in Karenia Brevis Bloom: Evidence of Morphological Plasticity?
Journal of Marine Science and Engineering Communication Brevetoxin-Producing Spherical Cells Present in Karenia brevis Bloom: Evidence of Morphological Plasticity? Lucie Novoveská *,† and Alison Robertson Department of Marine Sciences, University of South Alabama and Dauphin Island Sea Lab, 102 Bienville Blvd, Dauphin Island, AL 36528, USA; [email protected] * Correspondence: [email protected]; Tel: +44-752-124-8436 † Current address: Industrial Phycology, Unit 2 Axis, Hawkfield Business Park, Bristol BS14 0BY, UK. Received: 21 December 2018; Accepted: 18 January 2019; Published: 23 January 2019 Abstract: Spherical cells were detected in low salinity waters during a bloom of Karenia brevis in Alabama coastal waters. These balls resembled K. brevis in size and organelle appearance, contained similar concentration of brevetoxin, and occurred during ongoing K. brevis bloom. Based on the environmental conditions in which these cells were observed, we speculate that a rapid drop in salinity triggered the sphere formation in K. brevis. Brevetoxin concentrations were comparable between surface water samples containing typical and atypical cells ranging from 1 to 10 ng/mL brevetoxin-3 equivalents. Accurate identification and quantification of cell abundance in the water column is essential for routine monitoring of coastal waters, so misidentification of these spherical cells may result in significant underestimation of cell densities, and consequently, brevetoxin level. These potential discrepancies may negatively impact the quality of regulatory decisions and their impact on shellfish harvest area closures. We demonstrate that traditional monitoring based on microscopy alone is not sufficient for brevetoxin detection, and supporting toxin data is necessary to evaluate potential risk. Keywords: Karenia brevis; morphology; plasticity; sphere-shape cell; salinity; cryptic species; regulatory monitoring; shellfish harvest area Karenia brevis is a marine dinoflagellate which naturally produces a suite of neurotoxins known as brevetoxins. -

Redalyc.Especies De Dinoflagelados Atecados (Dinophyta) De La Costa
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Maciel-Baltazar, Ebodio; Hernández-Becerril, David U. Especies de dinoflagelados atecados (Dinophyta) de la costa de Chiapas, sur del Pacífico mexicano Revista de Biología Marina y Oceanografía, vol. 48, núm. 2, agosto-, 2013, pp. 245-259 Universidad de Valparaíso Viña del Mar, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=47928716005 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista de Biología Marina y Oceanografía Vol. 48, Nº2: 245-259, agosto 2013 Artículo Especies de dinoflagelados atecados (Dinophyta) de la costa de Chiapas, sur del Pacífico mexicano Species of athecate dinoflagellates (Dinophyta) from coasts of Chiapas, southern Mexican Pacific Ebodio Maciel-Baltazar1 y David U. Hernández-Becerril2 1Laboratorio Estatal de Salud Pública, Instituto de Salud del Estado de Chiapas, Blvd. Salomón Gonzales Blanco 3452, Tuxtla Gutiérrez, 29040, Chiapas, México 2Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apdo. postal 70-305, Ciudad Universitaria, Coyoacán, 04510, México, D.F., México. [email protected] Abstract.- Athecate dinoflagellates have been poorly studied in the plankton of Mexican marine waters, mainly because of their fragility, as they may become deformed using nets and strong fixatives. However, their biodiversity and ecological role might be important in the planktonic realm. As part of routine phytoplankton monitoring in the Chiapas coasts, Mexico, in the southern Mexican Pacific, samples were obtained during 2009 by net (20 μm mesh) in vertical hauls (up to 15 m), fixed with Lugol’s solution and studied by light microscope (bright field and phase contrast). -
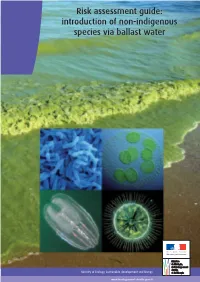
Risk Assessment Guide: Introduction of Non-Indigenous Species Via Ballast Water
Risk assessment guide: introduction of non-indigenous species via ballast water Contacts: Océane RIGNAULT & Damien CHEVALLIER Ministry of Ecology, Sustainable Development and Energy General Directorate of Infrastructures, Transport and the Sea Directorate of Maritime Affairs/Sub-directorate of maritime safety Phone: +33 (0)1 40 81 21 22 © Photo credit (cover): background photo: Blue-green algae at beach, Toxic algae bloom on Lake Erie in 2011 Tom Archer, handout The four small photos: Scanning electron microscope image of Vibrio cholerae bacteria, which infect the digestive system.Zeiss DSM 962 SEM ; Mnemiopsis leidyi (in the central Baltic Sea in January 2008), Jan-Erik Bruun, FIMR (Finnish Institute of Marine Research) ; Cyanobacteria, Josef Reischig / CC BY-SA 3.0 ; Lake-bottom-blanketing zebra mussel, Maria Antónia Sampayo. Acknowledgements to contributors: Perrine Prigent, Policy offi cer at the Directorate of Maritime Affairs, Laurent Guérin for the National Museum of Natural History, Daniel Masson for IFREMER, Frederic Quemmerais for the Agency for Marine Protected Areas, the Directorate of water and biodiversity, the Directorate of transport services. Ministèry of Ecology, Sustainable development and Energy General Directorate of Infrastructures, Transport and the Sea Directorate of Maritime Affairs Tour Séquoia - 92055 La Défense cedex Tél. : +33 (0)1 40 81 21 22 Layout-Design: SG/SPSSI/ATL2/Benoît Cudelou - Printing: SG/SPSSI/ATL2. Printed on paper certified European Ecolabel European certified paper on Printed SG/SPSSI/ATL2.