PDF Hosted at the Radboud Repository of the Radboud University Nijmegen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of Novel Pathways That Promote Anoikis Through Genome-Wide Screens
University of Massachusetts Medical School eScholarship@UMMS GSBS Dissertations and Theses Graduate School of Biomedical Sciences 2016-10-14 Identification of Novel Pathways that Promote Anoikis through Genome-wide Screens Victoria E. Pedanou University of Massachusetts Medical School Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/gsbs_diss Part of the Biology Commons, and the Cancer Biology Commons Repository Citation Pedanou VE. (2016). Identification of Novel Pathways that Promote Anoikis through Genome-wide Screens. GSBS Dissertations and Theses. https://doi.org/10.13028/M27G6D. Retrieved from https://escholarship.umassmed.edu/gsbs_diss/889 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in GSBS Dissertations and Theses by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. i TITLE PAGE IDENTIFICATION OF NOVEL PATHWAYS THAT PROMOTE ANOIKIS THROUGH GENOME-WIDE SCREENS A Dissertation Presented By VICTORIA ELIZABETH PEDANOU Submitted to the Faculty of the University of Massachusetts Graduate School of Biomedical Sciences, Worcester in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY OCTOBER 14TH, 2016 CANCER BIOLOGY ii SIGNATURE PAGE IDENTIFICATION OF NOVEL PATHWAYS THAT PROMOTE ANOIKIS THROUGH GENOME-WIDE SCREENS A Dissertation Presented By VICTORIA ELIZABETH PEDANOU This work was undertaken in the Graduate School of Biomedical Sciences Cancer Biology The signature of the Thesis Advisor signifies validation of Dissertation content ___________________________ Michael R. Green, Thesis Advisor The signatures of the Dissertation Defense Committee signify completion and approval as to style and content of the Dissertation __________________________________ Eric H. -

Leigh Syndrome: One Disorder, More Than 75 Monogenic Causes
Title: Leigh Syndrome: One disorder, more than 75 monogenic causes Running head: Leigh Syndrome: One disorder, many genes Author list: Nicole J. Lake, MSc,1,2 Alison G. Compton, PhD,1,2 Shamima Rahman, MD, PhD,3 and David R. Thorburn, PhD1,2,4 Affiliations: From the 1Murdoch Childrens Research Institute, Royal Children's Hospital, Melbourne, Victoria, Australia; 2Department of Paediatrics, The University of Melbourne, Melbourne, Victoria, Australia; 3Mitochondrial Research Group, Genetics and Genomic Medicine, Institute of Child Health, University College London and Metabolic Unit, Great Ormond Street Hospital, London, United Kingdom; 4Victorian Clinical Genetic Services, Royal Children's Hospital, Melbourne, Victoria, Australia. Corresponding author: Prof. David Thorburn Email [email protected] Telephone +61 3 8341 6235 Fax +61 3 8341 6212 Address Murdoch Childrens Research Institute, The Royal Children’s Hospital, 50 Flemington Rd, Parkville, Victoria, 3052, Australia Number of characters in the title and running head: 51 and 35, respectively Number of words in the abstract (107), and the body of manuscript not including abstract (4877) Number of figures (3), colour figures (1/3), and tables (3) 1 Abstract Leigh syndrome is the most common pediatric presentation of mitochondrial disease. This neurodegenerative disorder is genetically heterogeneous, and to date pathogenic mutations in more than 75 genes have been identified, encoded by two genomes (mitochondrial and nuclear). More than a third of these disease genes have been characterized in the last 5 years alone, reflecting the significant advances made in understanding its etiological basis. We review the diverse biochemical and genetic etiology of Leigh syndrome and associated clinical, neuroradiological and metabolic features that can provide clues for diagnosis. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Genetic Testing Policy Number: PG0041 ADVANTAGE | ELITE | HMO Last Review: 04/11/2021
Genetic Testing Policy Number: PG0041 ADVANTAGE | ELITE | HMO Last Review: 04/11/2021 INDIVIDUAL MARKETPLACE | PROMEDICA MEDICARE PLAN | PPO GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder terms, conditions, exclusions and limitations contract. It does not constitute a contract or guarantee regarding coverage or reimbursement/payment. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This medical policy is solely for guiding medical necessity and explaining correct procedure reporting used to assist in making coverage decisions and administering benefits. SCOPE X Professional X Facility DESCRIPTION A genetic test is the analysis of human DNA, RNA, chromosomes, proteins, or certain metabolites in order to detect alterations related to a heritable or acquired disorder. This can be accomplished by directly examining the DNA or RNA that makes up a gene (direct testing), looking at markers co-inherited with a disease-causing gene (linkage testing), assaying certain metabolites (biochemical testing), or examining the chromosomes (cytogenetic testing). Clinical genetic tests are those in which specimens are examined and results reported to the provider or patient for the purpose of diagnosis, prevention or treatment in the care of individual patients. Genetic testing is performed for a variety of intended uses: Diagnostic testing (to diagnose disease) Predictive -

From Gene Expression to the Clinic
UvA-DARE (Digital Academic Repository) Biomarker discovery for asthma phenotyping: From gene expression to the clinic Wagener, A.H. Publication date 2016 Document Version Final published version Link to publication Citation for published version (APA): Wagener, A. H. (2016). Biomarker discovery for asthma phenotyping: From gene expression to the clinic. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:02 Oct 2021 CHAPTER 4 Supporting Information File dsRNA-induced changes in gene expression pro les of primary nasal and bronchial epithelial cells from patients with asthma, rhinitis and controls methods Primary epithelial cell culture Epithelal cell cultures were done as previously described (1). Primary cells were obtained by first digesting the biopsies and brushes with collagenase 4 (Worthington Biochemi- cal Corp., Lakewood, NJ, USA) for 1 hour in Hanks’ balanced salt solution (Sigma-Aldrich, Zwijndrecht, The Netherlands). -

Metabolic Targets of Coenzyme Q10 in Mitochondria
antioxidants Review Metabolic Targets of Coenzyme Q10 in Mitochondria Agustín Hidalgo-Gutiérrez 1,2,*, Pilar González-García 1,2, María Elena Díaz-Casado 1,2, Eliana Barriocanal-Casado 1,2, Sergio López-Herrador 1,2, Catarina M. Quinzii 3 and Luis C. López 1,2,* 1 Departamento de Fisiología, Facultad de Medicina, Universidad de Granada, 18016 Granada, Spain; [email protected] (P.G.-G.); [email protected] (M.E.D.-C.); [email protected] (E.B.-C.); [email protected] (S.L.-H.) 2 Centro de Investigación Biomédica, Instituto de Biotecnología, Universidad de Granada, 18016 Granada, Spain 3 Department of Neurology, Columbia University Medical Center, New York, NY 10032, USA; [email protected] * Correspondence: [email protected] (A.H.-G.); [email protected] (L.C.L.); Tel.: +34-958-241-000 (ext. 20197) (L.C.L.) Abstract: Coenzyme Q10 (CoQ10) is classically viewed as an important endogenous antioxidant and key component of the mitochondrial respiratory chain. For this second function, CoQ molecules seem to be dynamically segmented in a pool attached and engulfed by the super-complexes I + III, and a free pool available for complex II or any other mitochondrial enzyme that uses CoQ as a cofactor. This CoQ-free pool is, therefore, used by enzymes that link the mitochondrial respiratory chain to other pathways, such as the pyrimidine de novo biosynthesis, fatty acid β-oxidation and amino acid catabolism, glycine metabolism, proline, glyoxylate and arginine metabolism, and sulfide oxidation Citation: Hidalgo-Gutiérrez, A.; metabolism. Some of these mitochondrial pathways are also connected to metabolic pathways González-García, P.; Díaz-Casado, in other compartments of the cell and, consequently, CoQ could indirectly modulate metabolic M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; pathways located outside the mitochondria. -
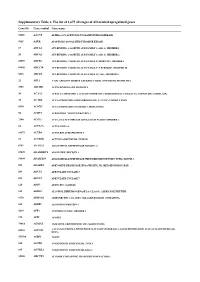
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces Cerevisiae
life Review Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces cerevisiae Leticia V. R. Franco 1,2,* , Luca Bremner 1 and Mario H. Barros 2 1 Department of Biological Sciences, Columbia University, New York, NY 10027, USA; [email protected] 2 Department of Microbiology,Institute of Biomedical Sciences, Universidade de Sao Paulo, Sao Paulo 05508-900, Brazil; [email protected] * Correspondence: [email protected] Received: 27 October 2020; Accepted: 19 November 2020; Published: 23 November 2020 Abstract: The ease with which the unicellular yeast Saccharomyces cerevisiae can be manipulated genetically and biochemically has established this organism as a good model for the study of human mitochondrial diseases. The combined use of biochemical and molecular genetic tools has been instrumental in elucidating the functions of numerous yeast nuclear gene products with human homologs that affect a large number of metabolic and biological processes, including those housed in mitochondria. These include structural and catalytic subunits of enzymes and protein factors that impinge on the biogenesis of the respiratory chain. This article will review what is currently known about the genetics and clinical phenotypes of mitochondrial diseases of the respiratory chain and ATP synthase, with special emphasis on the contribution of information gained from pet mutants with mutations in nuclear genes that impair mitochondrial respiration. Our intent is to provide the yeast mitochondrial specialist with basic knowledge of human mitochondrial pathologies and the human specialist with information on how genes that directly and indirectly affect respiration were identified and characterized in yeast. Keywords: mitochondrial diseases; respiratory chain; yeast; Saccharomyces cerevisiae; pet mutants 1. -
![PDSS2 Mouse Monoclonal Antibody [Clone ID: OTI4F9] Product Data](https://docslib.b-cdn.net/cover/8549/pdss2-mouse-monoclonal-antibody-clone-id-oti4f9-product-data-2018549.webp)
PDSS2 Mouse Monoclonal Antibody [Clone ID: OTI4F9] Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA503977 PDSS2 Mouse Monoclonal Antibody [Clone ID: OTI4F9] Product data: Product Type: Primary Antibodies Clone Name: OTI4F9 Applications: IF, IHC, WB Recommended Dilution: WB 1:2000, IHC 1:150, IF 1:100 Reactivity: Human, Mouse, Rat Host: Mouse Isotype: IgG2a Clonality: Monoclonal Immunogen: Full length human recombinant protein of human PDSS2(NP_065114) produced in HEK293T cell. Formulation: PBS (PH 7.3) containing 1% BSA, 50% glycerol and 0.02% sodium azide. Concentration: 0.98 mg/ml Purification: Purified from mouse ascites fluids or tissue culture supernatant by affinity chromatography (protein A/G) Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Predicted Protein Size: 43.9 kDa Gene Name: decaprenyl diphosphate synthase subunit 2 Database Link: NP_065114 Entrez Gene 71365 MouseEntrez Gene 365592 RatEntrez Gene 57107 Human Q86YH6 Background: The protein encoded by this gene is an enzyme that synthesizes the prenyl side-chain of coenzyme Q, or ubiquinone, one of the key elements in the respiratory chain. The gene product catalyzes the formation of all trans-polyprenyl pyrophosphates from isopentyl diphosphate in the assembly of polyisoprenoid side chains, the first step in coenzyme Q biosynthesis. Defects in this gene are a cause of coenzyme Q10 deficiency. COMPLETENESS: complete on the 3' end. This product is to be used for laboratory only. Not for diagnostic or therapeutic use. -

Coenzyme Q Biosynthesis in Health and Disease
Biochimica et Biophysica Acta 1857 (2016) 1079–1085 Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbabio Coenzyme Q biosynthesis in health and disease Manuel Jesús Acosta, Luis Vazquez Fonseca, Maria Andrea Desbats, Cristina Cerqua, Roberta Zordan, Eva Trevisson ⁎, Leonardo Salviati ⁎ Clinical Genetics Unit, Department of Woman and Child Health, University of Padova, and IRP Città della Speranza, Padova, Italy article info abstract Article history: Coenzyme Q (CoQ, or ubiquinone) is a remarkable lipid that plays an essential role in mitochondria as an electron Received 31 January 2016 shuttle between complexes I and II of the respiratory chain, and complex III. It is also a cofactor of other dehydro- Received in revised form 29 March 2016 genases, a modulator of the permeability transition pore and an essential antioxidant. Accepted 30 March 2016 CoQ is synthesized in mitochondria by a set of at least 12 proteins that form a multiprotein complex. The exact Available online 7 April 2016 composition of this complex is still unclear. Most of the genes involved in CoQ biosynthesis (COQ genes) have been studied in yeast and have mammalian orthologues. Some of them encode enzymes involved in the modifi- Keywords: Coenzyme Q cation of the quinone ring of CoQ, but for others the precise function is unknown. Two genes appear to have a Ubiquinone regulatory role: COQ8 (and its human counterparts ADCK3 and ADCK4) encodes a putative kinase, while PTC7 en- Coenzyme Q10 deficiency codes a phosphatase required for the activation of Coq7. Mitochondrial disorders Mutations in human COQ genes cause primary CoQ10 deficiency, a clinically heterogeneous mitochondrial disor- Steroid resistant nephrotic syndrome der with onset from birth to the seventh decade, and with clinical manifestation ranging from fatal multisystem disorders, to isolated encephalopathy or nephropathy. -

Directed Differentiation of Human Embryonic Stem Cells Into Haematopoietic and Definitive Endodermal Lineages
DIRECTED DIFFERENTIATION OF HUMAN EMBRYONIC STEM CELLS INTO HAEMATOPOIETIC AND DEFINITIVE ENDODERMAL LINEAGES ABRAHAM SUMAN MARY (M.Sc MICROBIOLOGY, UNIV. OF MUMBAI, INDIA) A THESIS SUBMITTED FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF BIOCHEMISTRY NATIONAL UNIVERSITY OF SINGAPORE 2009 ACKNOWLEDGEMENTS In all things I give YOU glory! You have always led me through amazing paths and given me gifts that I don’t deserve. I thank you Lord for all the blessings you constantly shower on me. Everything is possible with God! I thank Dr. Alan Colman for being my guide and helping me to initiate the work contained in this dissertation. His support and encouragement have been invaluable. Thank you, Alan for your support through the years. Dr. Norris Ray Dunn took me under his wing and guided me through this endeavour. He has been a true mentor, always willing to teach, and I have learned a lot from him. Ray, thank you for showing me the way and helping me reach this juncture. A significant part of the research was conducted at ES Cell International Pte Ltd to whom I would like to express my sincere gratitude. Triona, Jacqui, Robert, Michael, Bruce, Chirag, Suzan and so many others have played important roles and encouraged me at all times. Critical portions of this work were done at the Institute of Medical Biology, A*Star. I would like to register my appreciation for the support and help provided by many people in IMB especially, members of the Ray Dunn lab, Mike Jones lab and Alan Colman lab. Kee Yew, thank you for giving me your precious time and helping me with some of the most important data in this dissertation. -

Mitochondrial Diseases: Expanding the Diagnosis in the Era of Genetic Testing
Saneto. J Transl Genet Genom 2020;4:384-428 Journal of Translational DOI: 10.20517/jtgg.2020.40 Genetics and Genomics Review Open Access Mitochondrial diseases: expanding the diagnosis in the era of genetic testing Russell P. Saneto1,2 1Center for Integrative Brain Research, Neuroscience Institute, Seattle, WA 98101, USA. 2Department of Neurology/Division of Pediatric Neurology, Seattle Children’s Hospital/University of Washington, Seattle, WA 98105, USA. Correspondence to: Dr. Russell P. Saneto, Department of Neurology/Division of Pediatric Neurology, Seattle Children’s Hospital/ University of Washington, 4800 Sand Point Way NE, Seattle, WA 98105, USA. E-mail: [email protected] How to cite this article: Saneto RP. Mitochondrial diseases: expanding the diagnosis in the era of genetic testing. J Transl Genet Genom 2020;4:348-428. http://dx.doi.org/10.20517/jtgg.2020.40 Received: 29 Jun 2020 First Decision: 27 Jul 2020 Revised: 15 Aug 2020 Accepted: 21 Aug 2020 Available online: 29 Sep 2020 Academic Editor: Andrea L. Gropman Copy Editor: Cai-Hong Wang Production Editor: Jing Yu Abstract Mitochondrial diseases are clinically and genetically heterogeneous. These diseases were initially described a little over three decades ago. Limited diagnostic tools created disease descriptions based on clinical, biochemical analytes, neuroimaging, and muscle biopsy findings. This diagnostic mechanism continued to evolve detection of inherited oxidative phosphorylation disorders and expanded discovery of mitochondrial physiology over the next two decades. Limited genetic testing hampered the definitive diagnostic identification and breadth of diseases. Over the last decade, the development and incorporation of massive parallel sequencing has identified approximately 300 genes involved in mitochondrial disease.